S-benzylglutathione
Modify Date: 2025-09-13 23:11:18
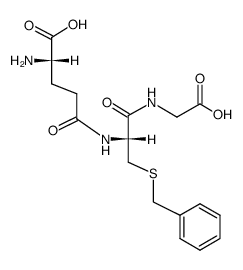
S-benzylglutathione structure
|
Common Name | S-benzylglutathione | ||
|---|---|---|---|---|
| CAS Number | 6803-17-4 | Molecular Weight | 397.44600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C17H23N3O6S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of S-benzylglutathioneS-Benzylglutathione is a competitive glutathionase inhibitor. S-Benzylglutathione is converted to the corresponding cysteine derivatives by rat kidney microsomes. S-Benzylglutathione can be used for the research of metabolic breakdown of glutathione by the glutathionase system[1]. |
| Name | γ-Glu-BzlCys-Gly |
|---|---|
| Synonym | More Synonyms |
| Description | S-Benzylglutathione is a competitive glutathionase inhibitor. S-Benzylglutathione is converted to the corresponding cysteine derivatives by rat kidney microsomes. S-Benzylglutathione can be used for the research of metabolic breakdown of glutathione by the glutathionase system[1]. |
|---|---|
| Related Catalog |
| Molecular Formula | C17H23N3O6S |
|---|---|
| Molecular Weight | 397.44600 |
| Exact Mass | 397.13100 |
| PSA | 184.12000 |
| LogP | 1.27960 |
| S-Benzyl-glutathion |
| N-(S-benzyl-N-γ-L-glutamyl-L-cysteinyl)-glycine |
| S-benzylglutathione |
| (S)-2-Amino-4-[(R)-2-benzylsulfanyl-1-(carboxymethyl-carbamoyl)-ethylcarbamoyl]-butyric acid |
| S-benzyl-L-glutathione |
| N-(S-Benzyl-N-γ-L-glutamyl-L-cysteinyl)-glycin |