Ethacrynic acid

Ethacrynic acid structure
|
Common Name | Ethacrynic acid | ||
|---|---|---|---|---|
| CAS Number | 58-54-8 | Molecular Weight | 303.13800 | |
| Density | 1.35g/cm3 | Boiling Point | 480ºC at 760mmHg | |
| Molecular Formula | C13H12Cl2O4 | Melting Point | 125 °C | |
| MSDS | Chinese USA | Flash Point | 244.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Ethacrynic acidEthacrynic acid is a diuretic. Ethacrynic acid is an inhibitor of glutathione S-transferases (GSTs). Ethacrynic acid is a potent inhibitor of NF-kB-signaling pathway, and also modulates leukotriene formation. Ethacrynic acid also inhibits L-type voltage-dependent and store-operated calcium channel, leading to relaxation of airway smooth muscle (ASM) cells. Ethacrynic acid has anti-inflammatory properties that reduces the retinoid-induced ear edema in mice[1][2][3][4]. |
| Name | etacrynic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Ethacrynic acid is a diuretic. Ethacrynic acid is an inhibitor of glutathione S-transferases (GSTs). Ethacrynic acid is a potent inhibitor of NF-kB-signaling pathway, and also modulates leukotriene formation. Ethacrynic acid also inhibits L-type voltage-dependent and store-operated calcium channel, leading to relaxation of airway smooth muscle (ASM) cells. Ethacrynic acid has anti-inflammatory properties that reduces the retinoid-induced ear edema in mice[1][2][3][4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.35g/cm3 |
|---|---|
| Boiling Point | 480ºC at 760mmHg |
| Melting Point | 125 °C |
| Molecular Formula | C13H12Cl2O4 |
| Molecular Weight | 303.13800 |
| Flash Point | 244.1ºC |
| Exact Mass | 302.01100 |
| PSA | 63.60000 |
| LogP | 3.60570 |
| InChIKey | AVOLMBLBETYQHX-UHFFFAOYSA-N |
| SMILES | C=C(CC)C(=O)c1ccc(OCC(=O)O)c(Cl)c1Cl |
| Storage condition | -20?C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332-H315-H319-H335 |
| Precautionary Statements | P261-P280-P301 + P312 + P330-P305 + P351 + P338 |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AG6600000 |
|
~% 
Ethacrynic acid CAS#:58-54-8 |
| Literature: Molecules, , vol. 14, # 1 p. 19 - 35 |
|
~% 
Ethacrynic acid CAS#:58-54-8 |
| Literature: US4952724 A1, ; |
|
~% 
Ethacrynic acid CAS#:58-54-8 |
| Literature: Comptes Rendus Chimie, , vol. 16, # 7 p. 660 - 664 |
|
~% 
Ethacrynic acid CAS#:58-54-8 |
| Literature: Comptes Rendus Chimie, , vol. 16, # 7 p. 660 - 664 |
|
CYP2E1-mediated oxidative stress regulates HO-1 and GST expression in maneb- and paraquat-treated rat polymorphonuclear leukocytes.
Mol. Cell Biochem. 393(1-2) , 209-22, (2014) Cytochrome P4502E1 (CYP2E1), glutathione-S-transferase A4-4 (GSTA4-4), and inducible nitric oxide synthase (iNOS) are implicated in maneb- and paraquat-induced toxicity leading to various pathological... |
|
|
Purification and characterization of the Staphylococcus aureus bacillithiol transferase BstA.
Biochim. Biophys. Acta 1840(9) , 2851-61, (2014) Gram-positive bacteria in the phylum Firmicutes synthesize the low molecular weight thiol bacillithiol rather than glutathione or mycothiol. The bacillithiol transferase YfiT from Bacillus subtilis wa... |
|
|
Differential involvement of glutathione S-transferase mu 1 and multidrug resistance protein 1 in melanoma acquired resistance to vinca alkaloids.
Fundam. Clin. Pharmacol. 29(1) , 62-71, (2015) On account of its extreme intrinsic resistance to apoptosis and of its strong ability to become chemoresistant after a primary response to drugs, malignant melanoma (MM) is still a therapeutic challen... |
| MFCD00056693 |
| Ethacrynic Acid |
| 2-[2,3-dichloro-4-(2-methylidenebutanoyl)phenoxy]acetic acid |
| EINECS 200-384-1 |
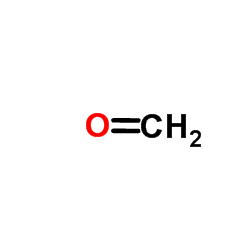
![Acetic acid, [2,3-dichloro-4-(1-oxobutyl)phenoxy]-, ethyl ester structure](https://image.chemsrc.com/caspic/468/2977-51-7.png)
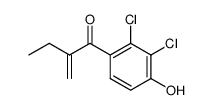
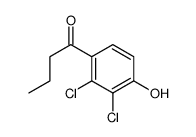
![[(6,7-Dichloro-2-ethyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]acetic acid structure](https://image.chemsrc.com/caspic/261/27366-21-8.png) CAS#:27366-21-8
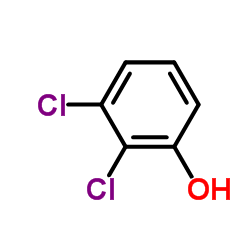
CAS#:27366-21-8 CAS#:1217-67-0
CAS#:1217-67-0![2-[2,3-dichloro-4-(2-methylbutanoyl)phenoxy]acetic acid structure](https://image.chemsrc.com/caspic/453/5378-94-9.png) CAS#:5378-94-9
CAS#:5378-94-9
