H-Tyr-ObzlTos
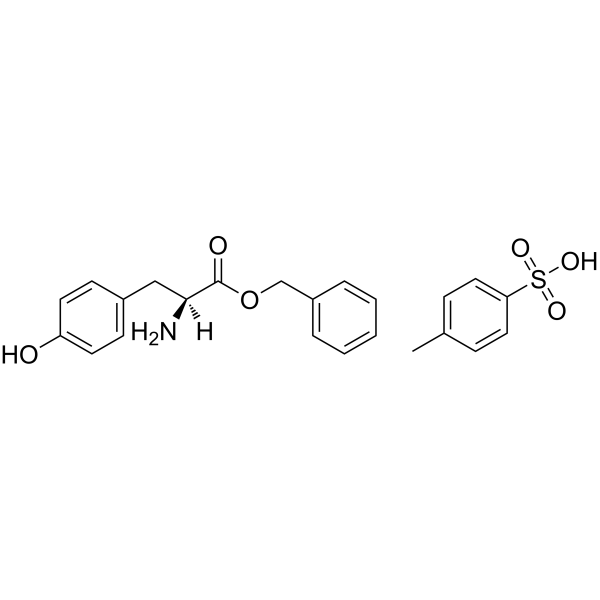
H-Tyr-ObzlTos structure
|
Common Name | H-Tyr-ObzlTos | ||
|---|---|---|---|---|
| CAS Number | 53587-11-4 | Molecular Weight | 443.513 | |
| Density | N/A | Boiling Point | 438.7ºC at 760 mmHg | |
| Molecular Formula | C23H25NO6S | Melting Point | 177 °C | |
| MSDS | USA | Flash Point | 359.8ºC | |
Use of H-Tyr-ObzlTosH-Tyr-OBzl.TosOH is a tyrosine derivative[1]. |
| Name | L-Tyrosine benzyl ester p-toluenesulfonate |
|---|---|
| Synonym | More Synonyms |
| Description | H-Tyr-OBzl.TosOH is a tyrosine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Boiling Point | 438.7ºC at 760 mmHg |
|---|---|
| Melting Point | 177 °C |
| Molecular Formula | C23H25NO6S |
| Molecular Weight | 443.513 |
| Flash Point | 359.8ºC |
| Exact Mass | 443.140259 |
| PSA | 135.30000 |
| LogP | 5.02820 |
| Index of Refraction | -13 ° (C=3, MeOH) |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~85% 
H-Tyr-ObzlTos CAS#:53587-11-4 |
| Literature: Sato, Atsushi; Yoshida, Masanori; Hara, Shoji Chemical Communications, 2008 , # 46 p. 6242 - 6244 |
|
~88% 
H-Tyr-ObzlTos CAS#:53587-11-4 |
| Literature: Arai, Isamu; Muramatsu, Ichiro Journal of Organic Chemistry, 1983 , vol. 48, # 1 p. 121 - 123 |
|
~% 
H-Tyr-ObzlTos CAS#:53587-11-4 |
| Literature: Letters in Organic Chemistry, , vol. 7, # 1 p. 39 - 44 |
|
Effect of protease inhibitors and substrates on 3,5,3'-triiodothyronine binding to rat liver nuclear receptors.
Endocr. Regul. 26 , 127-131, (1992) The effect of protease inhibitors N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) and N-carbobenzoxy-L-phenylalanine chloromethyl ketone (ZPCK) at concentrations ranging from 1.5 x 10(-6) mol/l to ... |
|
|
Endosperm-specific expression of tyramine N-hydroxycinnamoyltransferase and tyrosine decarboxylase from a single self-processing polypeptide produces high levels of tyramine derivatives in rice seeds.
Biotechnol. Lett. 31 , 911-915, (2009) The plant-specific tyramine derivatives, feruloyltyramine (FT) and 4-coumaroyltyramine (CT), represent bioactive compounds found at low levels in many plant species. We generated transgenic rice seeds... |
|
|
Purification, characterization and partial amino acid sequencing of hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase from tobacco cell-suspension cultures.
Eur. J. Biochem. 247 , 1127-1135, (1997) We report the purification of hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase (THT) to apparent homogeneity in 12% yield from tobacco (Nicotiana tabacum L. cv. Xanthi) cell-suspension cu... |
| H-Tyr-OBzl.TosOH |
| H-Tyr-OBzl·Tos-OH |
| MFCD00038959 |
| EINECS 258-650-8 |
| Benzyl tyrosinate 4-methylbenzenesulfonate (1:1) |
| L-Tyrosine Benzyl Ester p-Toluenesulfonate |
| Tyr-OBzl TosOH |
| L-Tyrosine, O-(phenylmethyl)-, 4-methylbenzenesulfonate (1:1) |
| O-Benzyl-L-tyrosine 4-methylbenzenesulfonate (1:1) |
| benzyl (2S)-2-amino-3-(4-hydroxyphenyl)propanoate,4-methylbenzenesulfonic acid |
| Tyrosine, phenylmethyl ester, 4-methylbenzenesulfonate (1:1) |
| (S)-Benzyl 2-amino-3-(4-hydroxyphenyl)propanoate 4-methylbenzenesulfonate |
| H-TYR-OBZL·TOS |
| H-Tyr-ObzlTos |
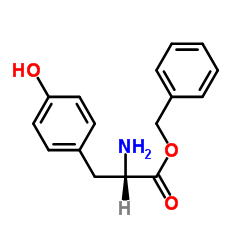
 CAS#:5513-40-6
CAS#:5513-40-6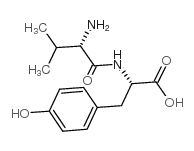 CAS#:3061-91-4
CAS#:3061-91-4 CAS#:69558-55-0
CAS#:69558-55-0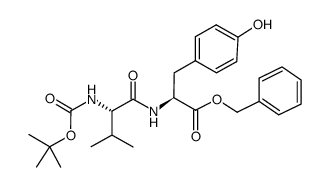 CAS#:75957-53-8
CAS#:75957-53-8
