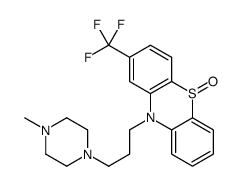
Trifluoperazine dihydrochloride

Trifluoperazine dihydrochloride structure
|
Common Name | Trifluoperazine dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 440-17-5 | Molecular Weight | 480.417 | |
| Density | N/A | Boiling Point | 506ºC at 760 mmHg | |
| Molecular Formula | C21H26Cl2F3N3S | Melting Point | 243 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 259.8ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Trifluoperazine dihydrochlorideTrifluoperazine Dihydrochloride is a potent dopamine D2 receptor inhibitor used as an antipsychotic and an antiemetic.Target: Dopamine D2 ReceptorTrifluoperazine Dihydrochloride is a potent dopamine D2 receptor inhibitor used as an antipsychotic and an antiemetic. Trifluoperazine inhibited in a dose-dependent manner the stimulation of glycogenolysis, gluconeogenesis, and ureogenesis due to alpha 1-adrenergic stimulation in rat hepatocytes. Trifluoperazine is much more potent at alpha 1- than at alpha 2-adrenergic receptors [1]. Trifluoperazine was not clearly different in terms of 'no substantial improvement' (n=1016, 27 RCTs, RR 1.06 CI 0.98 to 1.14) or leaving the study early (n=930, 22 RCTs, RR 1.15 CI 0.83 to 1.58). Almost identical numbers of people reported at least one adverse event (60%) in each group (n=585, 14 RCTs, RR 0.99 CI 0.87 to 1.13), although trifluoperazine was more likely to cause extrapyramidal adverse effects overall when compared to low potency antipsychotics such as chlorpromazine (n=130, 3 RCTs, RR 1.66 CI 1.03 to 2.67, NNH 6 CI 3 to 121). One small study (n=38) found no clear differences between trifluoperazine and the atypical drug, sulpiride [2]. |
| Name | trifluoperazine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Trifluoperazine Dihydrochloride is a potent dopamine D2 receptor inhibitor used as an antipsychotic and an antiemetic.Target: Dopamine D2 ReceptorTrifluoperazine Dihydrochloride is a potent dopamine D2 receptor inhibitor used as an antipsychotic and an antiemetic. Trifluoperazine inhibited in a dose-dependent manner the stimulation of glycogenolysis, gluconeogenesis, and ureogenesis due to alpha 1-adrenergic stimulation in rat hepatocytes. Trifluoperazine is much more potent at alpha 1- than at alpha 2-adrenergic receptors [1]. Trifluoperazine was not clearly different in terms of 'no substantial improvement' (n=1016, 27 RCTs, RR 1.06 CI 0.98 to 1.14) or leaving the study early (n=930, 22 RCTs, RR 1.15 CI 0.83 to 1.58). Almost identical numbers of people reported at least one adverse event (60%) in each group (n=585, 14 RCTs, RR 0.99 CI 0.87 to 1.13), although trifluoperazine was more likely to cause extrapyramidal adverse effects overall when compared to low potency antipsychotics such as chlorpromazine (n=130, 3 RCTs, RR 1.66 CI 1.03 to 2.67, NNH 6 CI 3 to 121). One small study (n=38) found no clear differences between trifluoperazine and the atypical drug, sulpiride [2]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 506ºC at 760 mmHg |
|---|---|
| Melting Point | 243 °C (dec.)(lit.) |
| Molecular Formula | C21H26Cl2F3N3S |
| Molecular Weight | 480.417 |
| Flash Point | 259.8ºC |
| Exact Mass | 479.117645 |
| PSA | 35.02000 |
| LogP | 6.49040 |
| Vapour Pressure | 2.32E-10mmHg at 25°C |
| InChIKey | BXDAOUXDMHXPDI-UHFFFAOYSA-N |
| SMILES | CN1CCN(CCCN2c3ccccc3Sc3ccc(C(F)(F)F)cc32)CC1.Cl.Cl |
| Storage condition | 2-8°C |
| Stability | Hygroscopic |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SP1750000 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
Antidepressants activate the lysophosphatidic acid receptor LPA(1) to induce insulin-like growth factor-I receptor transactivation, stimulation of ERK1/2 signaling and cell proliferation in CHO-K1 fibroblasts.
Biochem. Pharmacol. 95 , 311-23, (2015) Different lines of evidence indicate that the lysophosphatidic acid (LPA) receptor LPA1 is involved in neurogenesis, synaptic plasticity and anxiety-related behavior, but little is known on whether th... |
|
|
Evaluation of the in vitro/in vivo potential of five berries (bilberry, blueberry, cranberry, elderberry, and raspberry ketones) commonly used as herbal supplements to inhibit uridine diphospho-glucuronosyltransferase.
Food Chem. Toxicol. 72 , 13-9, (2014) In this study, we evaluated inhibitory potentials of popularly-consumed berries (bilberry, blueberry, cranberry, elderberry, and raspberry ketones) as herbal supplements on UGT1A1, UGT1A4, UGT1A6, UGT... |
|
|
Evaluation of thein vitro/in vivodrug interaction potential of BST204, a purified dry extract of ginseng, and its four bioactive ginsenosides through cytochrome P450 inhibition/induction and UDP-glucuronosyltransferase inhibition
Food Chem. Toxicol. 68 , 117-27, (2014) • BST204 is a purified dry extract of ginseng containing high amounts of Rh2 and Rg3. • BST204 had only weak inhibitory effects on nine CYPs and five UGTs. • It is unlikely that BST204 alter pharmacok... |
| Trifluoperazine Dihydrochloride |
| 2-Trifluoromethyl-10-[3-(1-methyl-4-piperazinyl)propyl]phenothiazine dihydrochloride |
| 10H-Phenothiazine, 10-[3- (4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-, dihydrochloride |
| Phenothiazine, 10-[3- (4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-, dihydrochloride |
| 10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine dihydrochloride |
| 10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine,dihydrochloride |
| MFCD00012656 |
| 10-[3-(4-Methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)-10H-phenothiazine dihydrochloride |
| EINECS 207-123-0 |
| 10H-phenothiazine, 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-, dihydrochloride |
| 10H-Phenothiazine, 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-, hydrochloride (1:2) |
| Eskazine dihydrochloride |
| Triflurin dihydrochloride |
| 10-[3-(4-Methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10H-phenothiazine dihydrochloride |
 CAS#:1549-88-8
CAS#:1549-88-8
