SM 7368
Modify Date: 2025-08-24 17:48:41
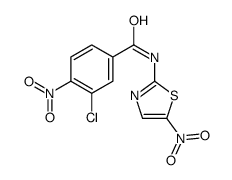
SM 7368 structure
|
Common Name | SM 7368 | ||
|---|---|---|---|---|
| CAS Number | 380623-76-7 | Molecular Weight | 328.68900 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C10H5ClN4O5S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of SM 7368SM-7368 is a potent NF-kB inhibitor that targets downstream of MAPK p38 activation[1]. SM-7368 inhibits TNF-α-induced MMP-9 upregulation. SM-7368 can be used for the research of chemotherapies targeting TNF-α-mediated tumor invasion and metastasis [2]. |
| Name | 3-Chloro-4-nitro-N-(5-nitro-1,3-thiazol-2-yl)benzamide |
|---|---|
| Synonym | More Synonyms |
| Description | SM-7368 is a potent NF-kB inhibitor that targets downstream of MAPK p38 activation[1]. SM-7368 inhibits TNF-α-induced MMP-9 upregulation. SM-7368 can be used for the research of chemotherapies targeting TNF-α-mediated tumor invasion and metastasis [2]. |
|---|---|
| Related Catalog | |
| Target |
NF-κB MMP-9 p38 MAP kinase |
| In Vitro | SM-7368 (5 μM) targets downstream of MAPK p38 activation in the human colon derived crypt like HT-29 and Caco-2 epithelial cell lines[1]. SM-7368 inhibits TNF-α-induced MMP-9 upregulation in a concentration-dependent manner and shows maximal activity at 10 μM. SM-7368 inhibits TNF-α-induced MMP-9 mRNA transcript accumulation and protein expression. SM-7368 strongly inhibits TNF-α-induced NF-κB activity but not AP-1 activity. SM-7368 strongly inhibits the TNF-α-induced invasion of HT1080 human fibrosarcoma cell line[2]. SM-7368 (10-25 μM) greatly inhibits TNF-α (20 ng/mL)-induced MMP-9 upregulation. 10 μM of SM-7368 almost completely abrogates this upregulation[2]. Western Blot Analysis[2] Cell Line: HT1080 human fibrosarcoma cells Concentration: 0, 1, 5, 10, 15, 20, and 25 μM Incubation Time: 24 hours Result: Greatly inhibited TNF-α (20 ng/mL)-induced MMP-9 upregulation in a concentration-dependent manner. |
| References |
| Molecular Formula | C10H5ClN4O5S |
|---|---|
| Molecular Weight | 328.68900 |
| Exact Mass | 327.96700 |
| PSA | 165.36000 |
| LogP | 4.29560 |
| InChIKey | XCHLNGBTHLJLFG-UHFFFAOYSA-N |
| SMILES | O=C(Nc1ncc([N+](=O)[O-])s1)c1ccc([N+](=O)[O-])c(Cl)c1 |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| NF-kappaB Activation Inhibitor III |
| SM-7368 |
| 3-Chloro-4-nitro-N-(5-nitro-2-thiazolyl)-benzamide |
| Benzamide,3-chloro-4-nitro-N-(5-nitro-2-thiazolyl) |
| 3-Chloro-4-nitro-N-(5-nitro-2-thiazolyl)-benzamide,SM 7368 |