Sodium 3-methyl-2-oxobutanoate
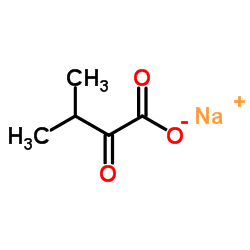
Sodium 3-methyl-2-oxobutanoate structure
|
Common Name | Sodium 3-methyl-2-oxobutanoate | ||
|---|---|---|---|---|
| CAS Number | 3715-29-5 | Molecular Weight | 138.097 | |
| Density | N/A | Boiling Point | 170.2ºC at 760 mmHg | |
| Molecular Formula | C5H7NaO3 | Melting Point | 220-230 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Sodium 3-methyl-2-oxobutanoateSodium 3-methyl-2-oxobutanoate is a precursor of pantothenic acid in Escherichia coli[1][2][3]. |
| Name | 3-Methyl-2-oxobutanoic acid, sodium salt |
|---|---|
| Synonym | More Synonyms |
| Description | Sodium 3-methyl-2-oxobutanoate is a precursor of pantothenic acid in Escherichia coli[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Sodium 3-methyl-2-oxobutanoate (alpha-Ketoisovaleric acid) is a precursor of pantothenic acid in Escherichia coli[1]. Sodium 3-methyl-2-oxobutanoate (alpha-Ketoisovaleric acid) enhances alpha-ketoisocaproic acid and alpha-keto-beta-methyl-n-valeric acid, but diminishes the corresponding amino acids, and causes an early decline of ornithine along with a late augmentation of plasma arginine[2]. |
| In Vivo | Sodium 3-methyl-2-oxobutanoate (alpha-Ketoisovaleric acid) induces convulsions through GABAergic and glutamatergic mechanisms in rats[3]. |
| References |
| Boiling Point | 170.2ºC at 760 mmHg |
|---|---|
| Melting Point | 220-230 °C (dec.)(lit.) |
| Molecular Formula | C5H7NaO3 |
| Molecular Weight | 138.097 |
| Exact Mass | 138.029282 |
| PSA | 57.20000 |
| InChIKey | WIQBZDCJCRFGKA-UHFFFAOYSA-M |
| SMILES | CC(C)C(=O)C(=O)[O-].[Na+] |
| Storage condition | Store at 0-5°C |
|
~% 
Sodium 3-methyl... CAS#:3715-29-5 |
| Literature: MERCK and CO., INC. Patent: WO2005/118529 A2, 2005 ; Location in patent: Page/Page column 26 ; |
|
~% 
Sodium 3-methyl... CAS#:3715-29-5 |
| Literature: Fizet Helvetica Chimica Acta, 1982 , vol. 65, # 7 p. 2024 - 2028 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
| HS Code | 2918300090 |
|---|---|
| Summary | 2918300090 other carboxylic acids with aldehyde or ketone function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Inhibition of brain energy metabolism by the alpha-keto acids accumulating in maple syrup urine disease.
Biochim. Biophys. Acta 1639(3) , 232-8, (2003) Neurological dysfunction is a common finding in patients with maple syrup urine disease (MSUD). However, the mechanisms underlying the neuropathology of brain damage in this disorder are poorly known.... |
|
|
Amino acid metabolism in the human fetus at term: leucine, valine, and methionine kinetics.
Pediatr. Res. 70(6) , 566-71, (2011) Human fetal metabolism is largely unexplored. Understanding how a healthy fetus achieves its fast growth rates could eventually play a pivotal role in improving future nutritional strategies for prema... |
|
|
Direct methods and residue type specific isotope labeling in NMR structure determination and model-driven sequential assignment.
J. Biomol. NMR 42(2) , 111-27, (2008) Direct methods in NMR based structure determination start from an unassigned ensemble of unconnected gaseous hydrogen atoms. Under favorable conditions they can produce low resolution structures of pr... |
| Butanoic acid, 3-methyl-2-oxo-, sodium salt (1:1) |
| MFCD00002581 |
| EINECS 223-062-2 |
| sodium,3-methyl-2-oxobutanoate |
| Butanoic acid, 3-methyl-2-oxo-, sodium salt |
| Sodium 3-methyl-2-oxobutanoate |
| 3-Methyl-2-oxobutanoic acid |

 CAS#:3952-67-8
CAS#:3952-67-8 CAS#:59935-29-4
CAS#:59935-29-4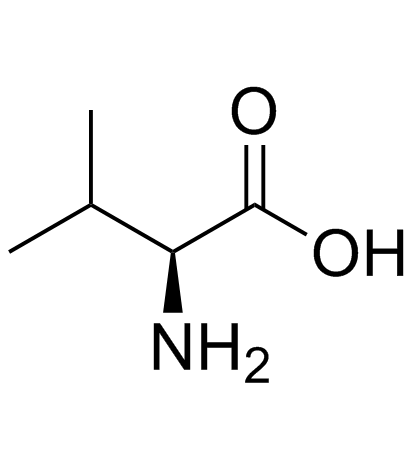 CAS#:72-18-4
CAS#:72-18-4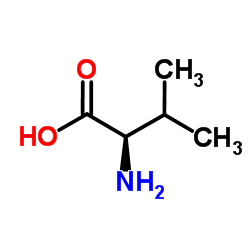 CAS#:640-68-6
CAS#:640-68-6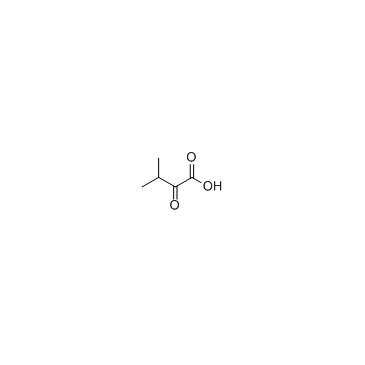 CAS#:759-05-7
CAS#:759-05-7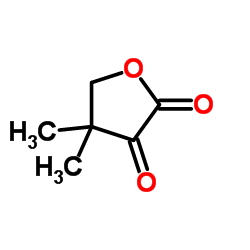 CAS#:13031-04-4
CAS#:13031-04-4 CAS#:79-50-5
CAS#:79-50-5 CAS#:76585-78-9
CAS#:76585-78-9![D-[2-2H]valine structure](https://image.chemsrc.com/caspic/042/77257-02-4.png) CAS#:77257-02-4
CAS#:77257-02-4 CAS#:77257-03-5
CAS#:77257-03-5
