4-Phenylbutan-1-ol
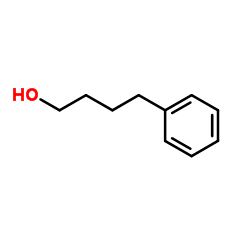
4-Phenylbutan-1-ol structure
|
Common Name | 4-Phenylbutan-1-ol | ||
|---|---|---|---|---|
| CAS Number | 3360-41-6 | Molecular Weight | 150.218 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 258.8±19.0 °C at 760 mmHg | |
| Molecular Formula | C10H14O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 114.3±17.3 °C | |
Use of 4-Phenylbutan-1-olBenzenebutanol is a component of an orally administrable antineoplastic agent. |
| Name | 4-phenylbutan-1-ol |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 258.8±19.0 °C at 760 mmHg |
| Molecular Formula | C10H14O |
| Molecular Weight | 150.218 |
| Flash Point | 114.3±17.3 °C |
| Exact Mass | 150.104462 |
| PSA | 20.23000 |
| LogP | 2.40 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.522 |
| Storage condition | 2~8°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | Xi: Irritant;C: Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | S23-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | 222-128-8 |
| HS Code | 29062900 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2906299090 |
|---|---|
| Summary | 2906299090 other aromatic alcohols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Cationic Xylene Tag for Increasing Sensitivity in Mass Spectrometry.
J. Am. Soc. Mass Spectrom. 26 , 1713-21, (2015) N-(2-(Bromomethyl)benzyl)-N,N-diethylethanaminium bromide, that we designate as CAX-B (cationic xylyl-bromide), is presented as a derivatization reagent for increasing sensitivity in mass spectrometry... |
|
|
NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel.
Br. J. Cancer 92(7) , 1240-6, (2005) Paclitaxel (PTX) is one of the most effective anticancer agents. In clinical practice, however, high incidences of adverse reactions of the drug, for example, neurotoxicity, myelosuppression, and alle... |
|
|
Cyclic ether formation in oxidations of primary alcohols by cerium (IV). Reactions of 5-phenyl-1-pentanol, 4-phenyl-1-butanol, and 3-phenyl-1-propanol with ceric ammonium nitrate. Doyle MP, et al.
J. Org. Chem. 40(10) , 1454-56, (1975)
|
| 1-Butanol,4-phenyl |
| 4-phenylbutan-1-ol |
| 1-phenyl-butan-4-ol |
| 4-Phenyl butanol-1 |
| 4-Phenyl-1-butanol |
| Q4R |
| 4-Phenylbutyl Alcohol |
| EINECS 222-128-8 |
| Phenylbutyl alcohol |
| 1-Butanol, 4-phenyl- (8CI) |
| 4-Phenylbutanol |
| Benzenebutanol |
| 1-Butanol, 4-phenyl- |
| MFCD00002971 |
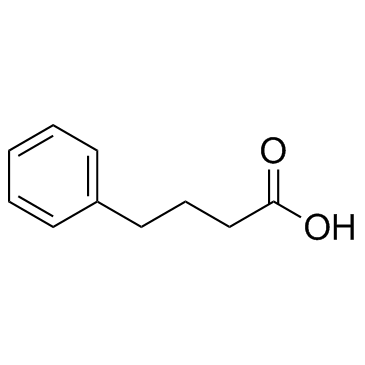 CAS#:1821-12-1
CAS#:1821-12-1 CAS#:16520-62-0
CAS#:16520-62-0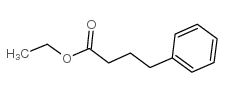 CAS#:10031-93-3
CAS#:10031-93-3![1-[4-(4-iodo-3-methoxy-3-methylbutoxy)butyl]benzene Structure](https://image.chemsrc.com/caspic/328/1141498-10-3.png) CAS#:1141498-10-3
CAS#:1141498-10-3 CAS#:1126-76-7
CAS#:1126-76-7 CAS#:7492-40-2
CAS#:7492-40-2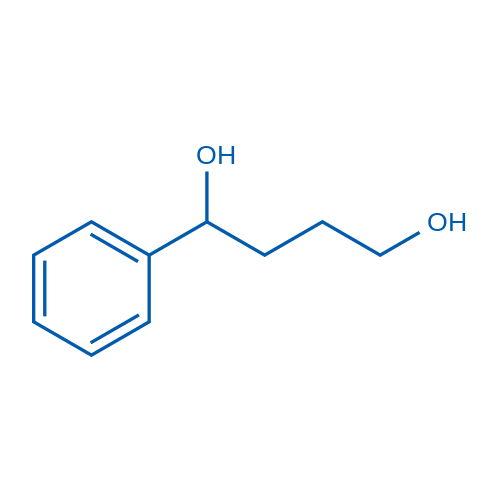 CAS#:4850-50-4
CAS#:4850-50-4 CAS#:300-57-2
CAS#:300-57-2 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:333742-34-0
CAS#:333742-34-0 CAS#:4830-93-7
CAS#:4830-93-7 CAS#:2046-18-6
CAS#:2046-18-6 CAS#:2051-95-8
CAS#:2051-95-8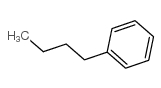 CAS#:104-51-8
CAS#:104-51-8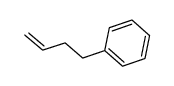 CAS#:768-56-9
CAS#:768-56-9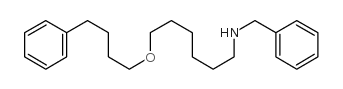 CAS#:97664-55-6
CAS#:97664-55-6 CAS#:119-64-2
CAS#:119-64-2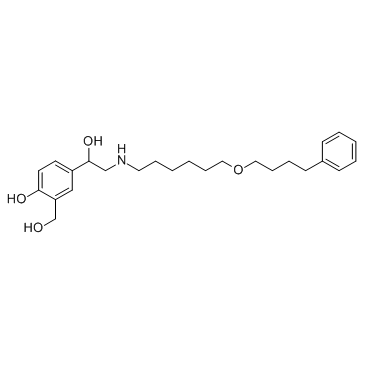 CAS#:89365-50-4
CAS#:89365-50-4 CAS#:17113-33-6
CAS#:17113-33-6
