2-(Trimethylsilyl)ethanol
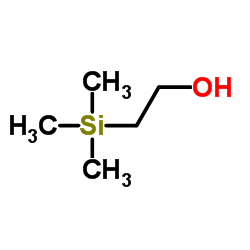
2-(Trimethylsilyl)ethanol structure
|
Common Name | 2-(Trimethylsilyl)ethanol | ||
|---|---|---|---|---|
| CAS Number | 2916-68-9 | Molecular Weight | 118.250 | |
| Density | 0.825 | Boiling Point | 145.0±13.0 °C at 760 mmHg | |
| Molecular Formula | C5H14OSi | Melting Point | 0ºC | |
| MSDS | USA | Flash Point | 50.6±0.0 °C | |
| Symbol |

GHS02 |
Signal Word | Warning | |
| Name | 2-(Trimethylsilyl)ethanol |
|---|---|
| Synonym | More Synonyms |
| Density | 0.825 |
|---|---|
| Boiling Point | 145.0±13.0 °C at 760 mmHg |
| Melting Point | 0ºC |
| Molecular Formula | C5H14OSi |
| Molecular Weight | 118.250 |
| Flash Point | 50.6±0.0 °C |
| Exact Mass | 118.081390 |
| PSA | 20.23000 |
| LogP | 1.36 |
| Vapour Pressure | 2.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.406 |
| InChIKey | ZNGINKJHQQQORD-UHFFFAOYSA-N |
| SMILES | C[Si](C)(C)CCO |
| Storage condition | 0-6°C |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS02 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H226 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R10;R36/37/38 |
| Safety Phrases | S16-S37/39-S26 |
| RIDADR | UN 1987 3/PG 3 |
| WGK Germany | 3 |
| RTECS | KM5480000 |
| Packaging Group | III |
| Hazard Class | 3 |
| HS Code | 29310095 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
|
Effects of charge, volume, and surface on binding of inhibitor and substrate moieties to acetylcholinesterase.
J. Med. Chem. 28(9) , 1309-13, (1985) Reversible inhibitors for acetylcholinesterase, AcChE, have been studied. Sterically similar alcohols with tetra-substituted uncharged beta groups, (CH3)3SiCH2CH2OH (I), (CH3)3CCH2CH2OH (IA), and CH3S... |
|
|
Generation of aza-ortho-xylylenes via ring opening of 2-(2-acylaminophenyl)aziridines: application in the construction of the communesin ring system.
Org. Lett. 8 , 3995, (2006) A new protocol for generating aza-ortho-xylylenes via acid-catalyzed or fluoride-promoted ring opening of 2-(2-acylaminophenyl)aziridines is described. This methodology has been exploited in the rapid... |
|
|
Silicon compounds as substrates and inhibitors of acetylcholinesterase.
Biochim. Biophys. Acta 791(2) , 278-80, (1984) Several trimethylsilyl derivatives were found to be ligands of acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7): trimethylsilylethyl acetate (III) and trimethylsilylmethyl acetate (V) ... |
| 2-Hydroxyethyltrimethylsilane |
| Silane, (2-hydroxyethyl)trimethyl- |
| (2-Hydroxyethyl)Trimethylsilane |
| 2-Trimethylsilylethanol |
| 2-(Trimethylsilyl)ethanal |
| 2-(trimethylsilyl)ethan-1-ol |
| MFCD00002825 |
| trimethsilylethanol |
| Ethanol, 2-(trimethylsilyl)- |
| trimethylsilyl ethanol |
| 2-(Trimethylsilyl)ethanol |
| 2-TMS-ethanol |
| Ethanol, 2- (trimethylsilyl)- |
| 2-Trimethylsilanyl-ethanol |
| EINECS 220-844-5 |
 CAS#:4071-88-9
CAS#:4071-88-9![B-[2-(trimethylsilyl)ethyl]9-borabicyclo[3.3.1]nonane Structure](https://image.chemsrc.com/caspic/407/72610-05-0.png) CAS#:72610-05-0
CAS#:72610-05-0 CAS#:3938-95-2
CAS#:3938-95-2 CAS#:754-05-2
CAS#:754-05-2 CAS#:75-16-1
CAS#:75-16-1 CAS#:50-00-0
CAS#:50-00-0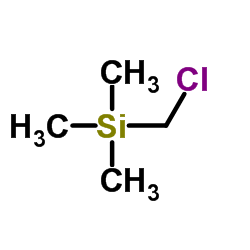 CAS#:2344-80-1
CAS#:2344-80-1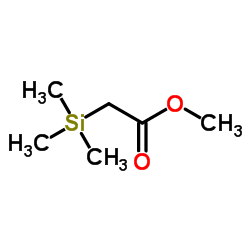 CAS#:2916-76-9
CAS#:2916-76-9![[(Trimethylsilyl)Methyl]Magnesium Chloride Structure](https://image.chemsrc.com/caspic/432/13170-43-9.png) CAS#:13170-43-9
CAS#:13170-43-9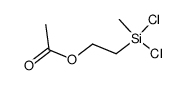 CAS#:5578-41-6
CAS#:5578-41-6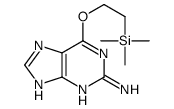 CAS#:141192-83-8
CAS#:141192-83-8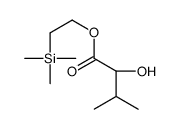 CAS#:185448-87-7
CAS#:185448-87-7 CAS#:184637-28-3
CAS#:184637-28-3 CAS#:201678-01-5
CAS#:201678-01-5 CAS#:75-76-3
CAS#:75-76-3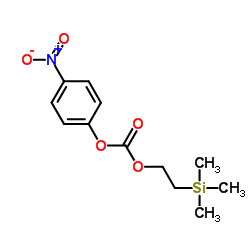 CAS#:80149-80-0
CAS#:80149-80-0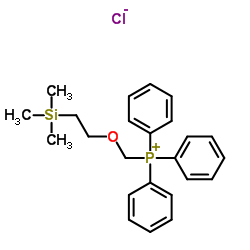 CAS#:82495-75-8
CAS#:82495-75-8 CAS#:78269-85-9
CAS#:78269-85-9 CAS#:1066-40-6
CAS#:1066-40-6 CAS#:54509-73-8
CAS#:54509-73-8
