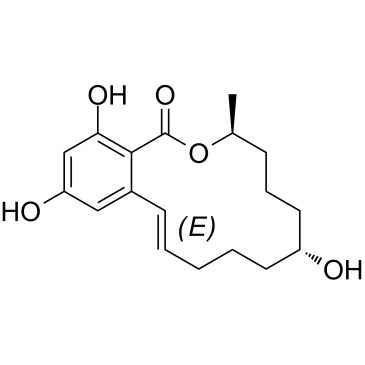
Zeranol
Zeranol structure
|
Common Name | Zeranol | ||
|---|---|---|---|---|
| CAS Number | 26538-44-3 | Molecular Weight | 322.396 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 576.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C18H26O5 | Melting Point | 178 - 185ºC | |
| MSDS | Chinese USA | Flash Point | 207.9±23.6 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of ZeranolZeranol, a metabolite of the mycoestrogen zearalenone, is an estrogen receptor agonist. Zeranol is used as a growth promoter of livestock due to its strong estrogenic activity[1][2]. |
| Name | (7R,11S)-7,15,17-trihydroxy-11-methyl-12-oxabicyclo[12.4.0]octadeca-1(14),15,17-trien-13-one |
|---|---|
| Synonym | More Synonyms |
| Description | Zeranol, a metabolite of the mycoestrogen zearalenone, is an estrogen receptor agonist. Zeranol is used as a growth promoter of livestock due to its strong estrogenic activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Zeranol exerts dose-dependent biphasic effects on ER-positive human breast carcinoma cells, accelerating cell growth at low concentrations and inducing apoptosis at high concentrations[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 576.0±50.0 °C at 760 mmHg |
| Melting Point | 178 - 185ºC |
| Molecular Formula | C18H26O5 |
| Molecular Weight | 322.396 |
| Flash Point | 207.9±23.6 °C |
| Exact Mass | 322.178009 |
| PSA | 86.99000 |
| LogP | 3.86 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.536 |
| InChIKey | DWTTZBARDOXEAM-GXTWGEPZSA-N |
| SMILES | CC1CCCC(O)CCCCCc2cc(O)cc(O)c2C(=O)O1 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302-H312-H319-H332 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R60;R36/37/38 |
| Safety Phrases | S53-S36/37/39-S45 |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 3 |
| RTECS | DM2520000 |
|
~45% 
Zeranol CAS#:26538-44-3 |
| Literature: INTERVET INTERNATIONAL B.V.; BETHELL, John, Richard; REID, Gary, Robert; AFFLECK, Krista, Marie; BREINING, Tibor Patent: WO2010/115478 A1, 2010 ; Location in patent: Page/Page column 22 ; |
|
~83% 
Zeranol CAS#:26538-44-3 |
| Literature: Snatzke; Angeli; Decorte; et al. Helvetica Chimica Acta, 1986 , vol. 69, # 3 p. 734 - 748 |
|
~% 
Zeranol CAS#:26538-44-3 |
| Literature: Snatzke; Angeli; Decorte; et al. Helvetica Chimica Acta, 1986 , vol. 69, # 3 p. 734 - 748 |
|
~% 
Zeranol CAS#:26538-44-3 |
| Literature: Gelo, Mirjana; Sunjic, Vitomir Tetrahedron, 1992 , vol. 48, # 31 p. 6511 - 6520 |
|
~% 
Zeranol CAS#:26538-44-3 |
| Literature: Gelo, Mirjana; Sunjic, Vitomir Tetrahedron, 1992 , vol. 48, # 31 p. 6511 - 6520 |
|
~% 
Zeranol CAS#:26538-44-3 |
| Literature: Hellwig, Veronika; Mayer-Bartschmid, Anke; Mueller, Hartwig; Greif, Gisela; Kleymann, Gerald; Zitzmann, Werner; Tichy, Hans-Volker; Stadler, Marc Journal of Natural Products, 2003 , vol. 66, # 6 p. 829 - 837 |
|
Simultaneous determination of six resorcylic acid lactones in feed using liquid chromatography-tandem mass spectrometry and multi-walled carbon nanotubes as a dispersive solid phase extraction sorbent.
J. Chromatogr. A. 1307 , 41-8, (2013) A simple and cost-effective pre-treatment procedure was developed for six resorcylic acid lactones (RALs) in feed using dispersive solid phase extraction (dSPE) with multi-walled carbon nanotubes (MWC... |
|
|
Effect of zeranol on expression of apoptotic and cell cycle proteins in murine placentae.
Toxicology 314(1) , 148-54, (2013) Mycotoxins are chemicals produced by fungus and many of them are toxic to humans. Zeranol is a mycotoxin used to promote growth in cattle in North America; yet such a practice draws concern about the ... |
|
|
Resorcylic acid lactones with cytotoxic and NF-κB inhibitory activities and their structure-activity relationships.
J. Nat. Prod. 74 , 1126-31, (2011) As part of our ongoing investigation of filamentous fungi for anticancer leads, an active fungal extract was identified from the Mycosynthetix library (MSX 63935; related to Phoma sp.). The initial ex... |
| EINECS 247-769-0 |
| 1H-2-Benzoxacyclotetradecin-1-one, 3,4,5,6,7,8,9,10,11,12-decahydro-7,14,16-trihydroxy-3-methyl-, (3S,7R)- |
| 6-(6,10-Dihydroxyundecyl)-β-resorcylic Acid μ-Lactone |
| (3S,7R)-3,4,5,6,7,8,9 |
| a-Zearalanol |
| Zeranol |
| Ralgro |
| (3S,7R)-3,4,5,6,7,8,9,10,11,12-Decahydro-7,14,16-trihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1-one |
| (3S,7R)-7,14,16-Trihydroxy-3-methyl-3,4,5,6,7,8,9,10,11,12-decahydro-1H-2-benzoxacyclotetradecin-1-one |
| α-zearalanol |
| MFCD00083519 |
| Ralone |
| Zearalanol |
| Ralabol |
| Zearanol |
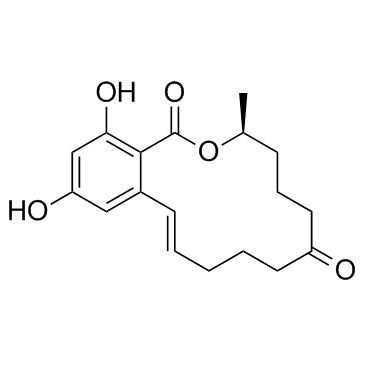

![2-[10(S)-Hydroxy-6-oxo-trans-1-undecenyl]-4,6-bis(benzyloxy)benzoic Acid μ-Lacton structure](https://image.chemsrc.com/caspic/055/34297-69-3.png)

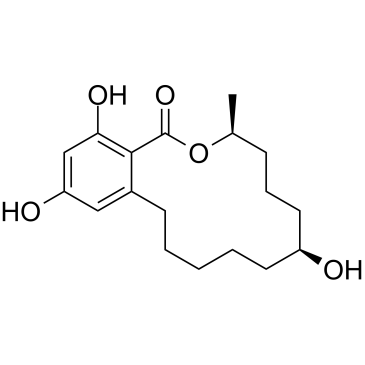
![(3S)-14,16-dihydroxy-3-methyl-1-oxo-3,4,5,6,7,8,9,10,11,12-decahydro-1H-benzo[c][1]oxacyclotetradecin-7-yl acetate structure](https://image.chemsrc.com/caspic/323/37786-44-0.png)