5-Carboxy-2-thiouracil
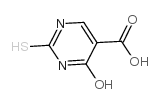
5-Carboxy-2-thiouracil structure
|
Common Name | 5-Carboxy-2-thiouracil | ||
|---|---|---|---|---|
| CAS Number | 23945-50-8 | Molecular Weight | 172.16200 | |
| Density | 1.74g/cm3 | Boiling Point | 461.2ºC at 760mmHg | |
| Molecular Formula | C5H4N2O3S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 232.7ºC | |
| Name | 2-thiouracil-5-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.74g/cm3 |
|---|---|
| Boiling Point | 461.2ºC at 760mmHg |
| Molecular Formula | C5H4N2O3S |
| Molecular Weight | 172.16200 |
| Flash Point | 232.7ºC |
| Exact Mass | 171.99400 |
| PSA | 122.11000 |
| LogP | 0.16910 |
| Vapour Pressure | 2.65E-09mmHg at 25°C |
| Index of Refraction | 1.719 |
| InChIKey | XKHWTDCFQVKNHW-UHFFFAOYSA-N |
| SMILES | O=C(O)c1c[nH]c(=S)[nH]c1=O |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933599090 |
|
~% 
5-Carboxy-2-thi... CAS#:23945-50-8 |
| Literature: TOKUYAMA CORPORATION Patent: EP802194 A3, 1997 ; |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis, characterization and antitumour activity of metal complexes of 5-carboxy-2-thiouracil.
Met. Based Drugs 5 , 35-39, (1998) Metal complexes of 5-carboxy-2-thiouracil with Mn(ll), Co(ll), Ni(ll), Cu(ll), Zn(ll) and Cd(ll) ions were synthesized, characterized, and subjected to a screening system for evaluation of antitumour ... |
|
|
[Synthesis and antibacterial and antitumoral activity of some methylhydrazonium salts of pyrimidine bases and their analogues (author's transl)].
Pharmazie 37 , 355-356, (1982) On reacting pyrimidine metabolites containing a carboxyl or sulfhydryl group with methylhydrazine or N-benzyl-N'-methylhydrazine, various methylhydrazonium salts of these metabolites were synthetized,... |
| 4-Oxo-2-thioxo-1,2,3,4-tetrahydro-pyrimidin-5-carbonsaeure |
| 5-Carboxy-2-thiouracil,2-Thiouracil-5-carboxylic acid,5-Carboxy-4-hydroxy-2-thiopyrimidine |
| 1,2,3,4-tetrahydro-4-oxo-2-thioxo-5-pyrimidinecarboxylicaci |
| 2-thioisoorotic acid |
| 2-Thiouracil-5-carboxylic acid,5-Carboxy-4-hydroxy-2-thiopyrimidine |
| 5-Carboxy-2-thiouracil |
| 1,2,3,4-Tetrahydro-4-oxo-2-thioxo-5-pyrimidinecarboxylic acid |
| 5-Carboxy-7-thiouracil |
| 5-carboxy-2-thio-uracil |
| 1,2,3,4-tetrahydro-4-oxo-2-thioxopyrimidine-5-carboxylic acid |
| 4-oxo-2-thioxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylic acid |
| 5-carboxy-2-thiouracil free acid |
| 5-CARBOXY-4-HYDROXY-2-THIOPYRIMIDINE |