NF 279
Modify Date: 2025-08-27 12:23:06
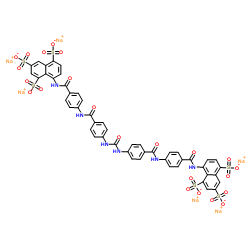
NF 279 structure
|
Common Name | NF 279 | ||
|---|---|---|---|---|
| CAS Number | 202983-32-2 | Molecular Weight | 1401.118 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C49H30N6Na6O23S6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of NF 279NF279 is a potent selective and reversible P2X1 receptor antagonist, with an IC50 of 19 nM. NF279 displays good selectivity over P2X2, P2X3 (IC50=1.62 μM), P2X4 (IC50>300 μM). NF279 is a dual HIV-1 coreceptor inhibitor that interferes with the functional engagement of CCR5 and CXCR4 by Env[1][2]. |
| Name | N-[4,6,8-tris(methylsulfonyl)naphthalen-1-yl]-4-[[4-[[4-[[4-[[4,6,8-tris(methylsulfonyl)naphthalen-1-yl]carbamoyl]phenyl]carbamoyl]phenyl]carbamoylamino]benzoyl]amino]benzamide |
|---|---|
| Synonym | More Synonyms |
| Description | NF279 is a potent selective and reversible P2X1 receptor antagonist, with an IC50 of 19 nM. NF279 displays good selectivity over P2X2, P2X3 (IC50=1.62 μM), P2X4 (IC50>300 μM). NF279 is a dual HIV-1 coreceptor inhibitor that interferes with the functional engagement of CCR5 and CXCR4 by Env[1][2]. |
|---|---|
| Related Catalog | |
| Target |
HIV |
| References |
| Molecular Formula | C49H30N6Na6O23S6 |
|---|---|
| Molecular Weight | 1401.118 |
| Exact Mass | 1399.907227 |
| PSA | 551.01000 |
| LogP | 10.99360 |
| Storage condition | -20°C |
| cc-368 |
| 1,3,5-Naphthalenetrisulfonic acid, 8,8'-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-, sodium salt (1:6) |
| Hexasodium 8-[(4-{[4-({[4-({4-[(4,6,8-trisulfonato-1-naphthyl)carbamoyl]phenyl}carbamoyl)phenyl]carbamoyl}amino)benzoyl]amino}benzoyl)amino]naphthalene-1,3,5-trisulfonate |
| Hexasodium 8,8'-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]di(1,3,5-naphthalenetrisulfonate) |
| NF 279 |