OBE022
Modify Date: 2025-10-23 19:36:54
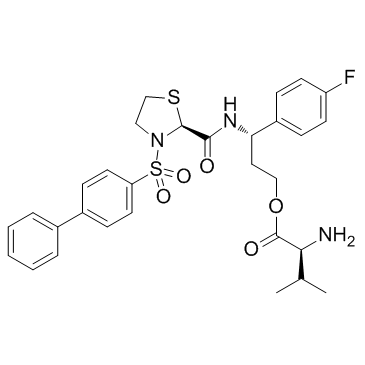
OBE022 structure
|
Common Name | OBE022 | ||
|---|---|---|---|---|
| CAS Number | 2005486-31-5 | Molecular Weight | 599.74 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C30H34FN3O5S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of OBE022OBE022 is an oral and selective prostaglandin F2α (PGF2α) receptor antagonist, with Kis of 1 nM, 26 nM for human and rat FP receptors, respectively. |
| Name | OBE022 |
|---|
| Description | OBE022 is an oral and selective prostaglandin F2α (PGF2α) receptor antagonist, with Kis of 1 nM, 26 nM for human and rat FP receptors, respectively. |
|---|---|
| Related Catalog | |
| Target |
Human FP Receptor:1 nM (Ki) Rat FP Receptor:26 nM (Ki) |
| In Vitro | OBE022 and OBE002 are assayed for FP binding affinity by competitive binding analysis with 3H-PGF2α using HEK293 cells stably transfected with the FP receptor. Binding affinities (Ki) of OBE022 for the human and rat FP receptor are 1 nM and 26 nM respectively. For OBE002, Kis are 6 nM for the human and 313 nM for the rat FP receptor. The binding of both OBE022 and OBE002 is reversible and competitive since increasing concentrations of either compound causes successive decreases in the slope of the binding curves, consistent with an increase in equilibrium dissociation constant (KD) without a reduction in receptor density[1]. |
| In Vivo | Time-course of the cumulative percentage of delivers mice after RU486-induced preterm parturition at GD17, in OBE022, nifedipine or vehicle treatment groups. Oral treatment with OBE022 delays the preterm birth caused by RU486 administration as reflected by a shift to the right of the percentage of delivery curve. The effect of oral treatment with nifedipine is comparable. Both OBE022 and nifedipine show a trend to increase the time of first pup delivery. As an important consequence of the prolongation of gestation, dams deliver viable pups. Combination of OBE022 and nifedipine cause a synergistic effect on the delay of RU486-induced preterm birth as reflected by a more pronounced shift to the right of the percentage of delivery curve, in comparison to OBE022 or nifedipine alone. Also, a larger increase of the time of first pup delivery is observed[1]. |
| Animal Admin | Mice[1] Primigravid CD1 mice, on day 17 of pregnancy (about 85% gestation) at the beginning of the experiments, are used. Approximately 3 hours before induction of preterm labor (Day 1 (D1) at 10h00), the pregnant mice at gestational day 17, are placed in individual cages with food and water ad libitum. Pregnant mice receive on D1 (at day time: 13h00) a single subcutaneous (s.c.) injection of RU486 at a dose of 2.5 mg/kg in a final volume of 10 mL/kg of sesame oil. OBE022 (10, 30 and 100 mg/kg) or nifedipine (5 mg/kg) are administered orally (p.o.) at a volume of 5 mL/kg once on D1 (18h00), twice on D2 (8h00 and 18h00) and once on D3 (8h00) for a total of 4 administrations. For combination treatment, mice receive OBE022 plus nifedipine using the same experimental design as single treatment[1]. |
| References |
| Molecular Formula | C30H34FN3O5S2 |
|---|---|
| Molecular Weight | 599.74 |
| InChIKey | UUIBKACUTXYSAK-YCVJPRETSA-N |
| SMILES | CC(C)C(N)C(=O)OCCC(NC(=O)C1SCCN1S(=O)(=O)c1ccc(-c2ccccc2)cc1)c1ccc(F)cc1 |