Leucomycin A1
Modify Date: 2025-08-31 15:37:51
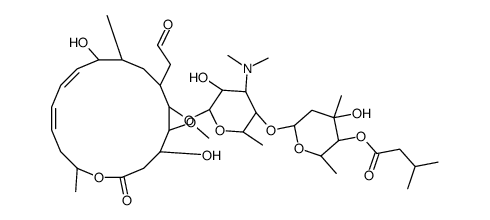
Leucomycin A1 structure
|
Common Name | Leucomycin A1 | ||
|---|---|---|---|---|
| CAS Number | 16846-34-7 | Molecular Weight | 785.96 | |
| Density | 1.21g/cm3 | Boiling Point | 874.7ºC at 760 mmHg | |
| Molecular Formula | C40H67NO14 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 482.8ºC | |
Use of Leucomycin A1Leucomycin A1 is a main component of the leucomycin complex produced by Streptomyces kitasatoensis, Leucomycin A1 is an antibiotic[1]. |
| Name | Leucomycin-A1 |
|---|---|
| Synonym | More Synonyms |
| Description | Leucomycin A1 is a main component of the leucomycin complex produced by Streptomyces kitasatoensis, Leucomycin A1 is an antibiotic[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.21g/cm3 |
|---|---|
| Boiling Point | 874.7ºC at 760 mmHg |
| Molecular Formula | C40H67NO14 |
| Molecular Weight | 785.96 |
| Flash Point | 482.8ºC |
| Exact Mass | 785.45600 |
| PSA | 199.98000 |
| LogP | 2.44260 |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.541 |
| Leucomycin V,3,4(sup B)-diacetate |
| Turimycin A2 |
| Leucomycin A8 |
| Leukomycin A8 |