Lixivaptan
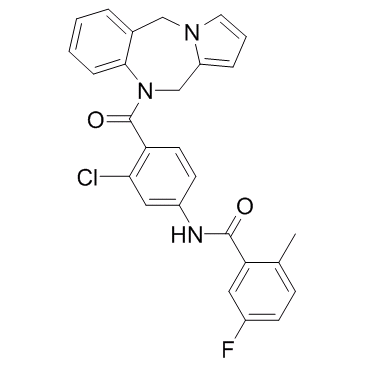
Lixivaptan structure
|
Common Name | Lixivaptan | ||
|---|---|---|---|---|
| CAS Number | 168079-32-1 | Molecular Weight | 473.926 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 626.5±55.0 °C at 760 mmHg | |
| Molecular Formula | C27H21ClFN3O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 332.7±31.5 °C | |
Use of LixivaptanLixivaptan (VPA-985, WAY-VPA 985) is an orally active and selective vasopressin receptor V2 antagonist, with IC50 values of 1.2 and 2.3 nM for human and rat V2, respectively. |
| Name | N-[3-chloro-4-(6,11-dihydropyrrolo[2,1-c][1,4]benzodiazepine-5-carbonyl)phenyl]-5-fluoro-2-methylbenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | Lixivaptan (VPA-985, WAY-VPA 985) is an orally active and selective vasopressin receptor V2 antagonist, with IC50 values of 1.2 and 2.3 nM for human and rat V2, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 1.2 nM (human V2), 2.3 nM (rat V2)[1] |
| In Vitro | Lixivaptan displays competitive antagonist activity at V2 receptors[1]. |
| In Vivo | In conscious dogs, water-loaded with 30 mL/kg (po) and arginine vasopressin (AVP)-treated (0.4 µg/kg in oil, sc), lixivaptan (1, 3, and 10 mg/kg po) increases Uvol over the AVP-treated vehicle group by 438, 1018, and 1133%, respectively, while Uosm decreases from 1222 mOsm/kg (water-loaded and AVP treated vehicle) to 307, 221, and 175 mOsm/kg, respectively. In homozygous Brattleboro rats lacking AVP, lixivaptan at 10 mg/kg po (i.e., 10 times the dose producing V2 antagonist activity) b.i.d. for 5 days, shows a sustained antagonist action without evidence of agonist effects. In a randomized double-blind placebo-controlled ascending single dose study, patients (deprived of fluids overnight before dosing) are dosed orally with 30, 75, or 150 mg of lixivaptan. All three doses increase urine flow and serum sodium concentrations and produced significant dose-related decreases in urinary osmolality[1]. Phase II clinical trials in patients with congestive heart failure, liver cirrhosis with ascites or syndrome of inappropriate antidiuretic hormone have demonstrated that lixivaptan increases water clearance without affecting renal sodium excretion or activating the neurohormonal system[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 626.5±55.0 °C at 760 mmHg |
| Molecular Formula | C27H21ClFN3O2 |
| Molecular Weight | 473.926 |
| Flash Point | 332.7±31.5 °C |
| Exact Mass | 473.130646 |
| PSA | 57.83000 |
| LogP | 7.23 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.658 |
| InChIKey | PPHTXRNHTVLQED-UHFFFAOYSA-N |
| SMILES | Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc32)c(Cl)c1 |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
Lixivaptan CAS#:168079-32-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 14 p. 2442 - 2444 |
|
~% 
Lixivaptan CAS#:168079-32-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 14 p. 2442 - 2444 |
|
~% 
Lixivaptan CAS#:168079-32-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 14 p. 2442 - 2444 |
|
~% 
Lixivaptan CAS#:168079-32-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 14 p. 2442 - 2444 |
|
~% 
Lixivaptan CAS#:168079-32-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 14 p. 2442 - 2444 |
| N-[3-Chloro-4-(5H-pyrrolo[2,1-c][1,4]benzodiazepin-10(11H)-ylcarbonyl)phenyl]-5-fluoro-2-methylbenzamide |
| WAY-VPA-985 |
| VPA-985 |
| Benzamide, N-[3-chloro-4-(5H-pyrrolo[2,1-c][1,4]benzodiazepin-10(11H)-ylcarbonyl)phenyl]-5-fluoro-2-methyl- |
| lixivaptan |
| UNII-8F5X4B082E |
![10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a][1,4]diazepine structure](https://image.chemsrc.com/caspic/297/22162-53-4.png)
![Benzoyl chloride, 2-chloro-4-[(5-fluoro-2-methylbenzoyl)amino]- structure](https://www.chemsrc.com/extcaspic/433/168080-80-6.png)