Vicine
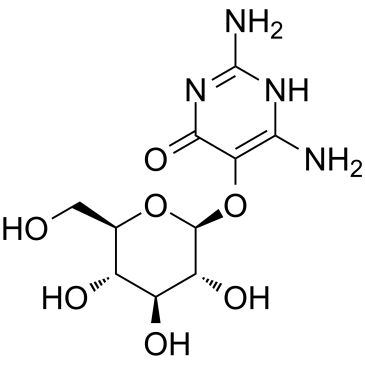
Vicine structure
|
Common Name | Vicine | ||
|---|---|---|---|---|
| CAS Number | 152-93-2 | Molecular Weight | 304.257 | |
| Density | 2.2±0.1 g/cm3 | Boiling Point | 627.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C10H16N4O7 | Melting Point | 242-244℃ | |
| MSDS | USA | Flash Point | 333.2±34.3 °C | |
Use of VicineVicine, an alkaloid glycoside found mainly in fava beans, is toxic in individuals and may cause haemolytic anaemia[1]. |
| Name | Glucopyranoside, divicine-5, .β.-D |
|---|---|
| Synonym | More Synonyms |
| Description | Vicine, an alkaloid glycoside found mainly in fava beans, is toxic in individuals and may cause haemolytic anaemia[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 2.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 627.4±65.0 °C at 760 mmHg |
| Melting Point | 242-244℃ |
| Molecular Formula | C10H16N4O7 |
| Molecular Weight | 304.257 |
| Flash Point | 333.2±34.3 °C |
| Exact Mass | 304.101898 |
| PSA | 197.17000 |
| LogP | -2.55 |
| Vapour Pressure | 0.0±4.2 mmHg at 25°C |
| Index of Refraction | 1.820 |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
Vicine CAS#:152-93-2 |
| Literature: Kunesch, Nicole; Miet, Christine; Poisson, Jacques Liebigs Annalen der Chemie, 1994 , # 11 p. 1059 - 1064 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties.
Int. J. Food Microbiol. 193 , 34-42, (2015) The effects of air classification and lactic acid bacteria fermentation on the reduction of anti-nutritional factors (vicine and convicine, trypsin inhibitor activity, condensed tannins and phytic aci... |
|
|
Vicianin, prunasin, and beta-cyanoalanine in common vetch seed as sources of urinary thiocyanate in the rat.
J. Agric. Food Chem. 49(10) , 5075-80, (2001) When young rats were fed a diet containing common vetch seed for 1 month, they excreted in the urine approximately 7 times more thiocyanate than they had ingested. Vicianin, prunasin, and beta-cyanoal... |
|
|
Biocatalytic production of alpha-hydroxy ketones and vicinal diols by yeast and human aldo-keto reductases.
Chem. Biol. Interact. 202(1-3) , 195-203, (2013) The α-hydroxy ketones are used as building blocks for compounds of pharmaceutical interest (such as antidepressants, HIV-protease inhibitors and antitumorals). They can be obtained by the action of en... |
| 2,6-Diamino-5-(β-D-glucopyranosyloxy)-4(1H)-pyrimidinone |
| 2,4-Diamino-6-oxo-1,6-dihydro-5-pyrimidinyl β-D-glucopyranoside |
| 2,6-Diamino-5-(β-D-glucopyranosyloxy)-(1H)-pyrimidin-4-one |
| 2,4-Diamino-6-oxypyrimidine-5-(b-D-glucopyranoside) |
| Vicine |
| Divicine 5-Glucoside |
| 2,4-Diamino-6-oxo-1,6-dihydropyrimidin-5-yl β-D-glucopyranoside |
| Vicioside |
| 4(3H)-pyrimidinone, 2,6-diamino-5-(β-D-glucopyranosyloxy)- |
| Divicine-b-glucoside |
| 4(1H)-Pyrimidinone, 2,6-diamino-5-(β-D-glucopyranosyloxy)- |
| EINECS 205-809-4 |
| Vicin |
| 2,6-Diamino-5-(b-D-glucopyranosyloxy)-4(1H)-pyrimidinone |
![ethyl (R)-2-cyano-2-[O-(2,3,4,6-tetra-O-benzyl-β-D-glucopyranosyloxy)]acetate structure](https://image.chemsrc.com/caspic/434/160812-25-9.png)

