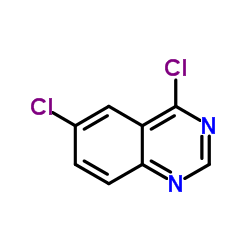
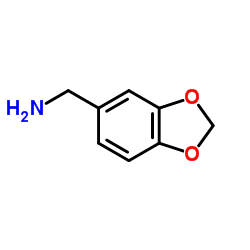
MBCQ

MBCQ structure
|
Common Name | MBCQ | ||
|---|---|---|---|---|
| CAS Number | 150450-53-6 | Molecular Weight | 313.73800 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H12ClN3O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of MBCQMBCQ is a potent and selective cGMP-specific phosphodiesterase (PDE V; PDE5) inhibitor with an IC50 of 19 nM. MBCQ lacks inhibitory activity toward other PDE isozymes (all IC50s>100 μM). MBCQ dilates coronary arteries via specific inhibition of cGMP-PDE[1][2][3]. |
| Name | N-(1,3-benzodioxol-5-ylmethyl)-6-chloroquinazolin-4-amine |
|---|---|
| Synonym | More Synonyms |
| Description | MBCQ is a potent and selective cGMP-specific phosphodiesterase (PDE V; PDE5) inhibitor with an IC50 of 19 nM. MBCQ lacks inhibitory activity toward other PDE isozymes (all IC50s>100 μM). MBCQ dilates coronary arteries via specific inhibition of cGMP-PDE[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
PDE V:19 nM (IC50) PDE Ⅰ:>100 μM (IC50) PDE Ⅱ:>100 μM (IC50) PDE Ⅲ:>100 μM (IC50) PDE Ⅳ:>100 μM (IC50) |
| In Vitro | MBCQ (compound 3d) induces relaxation of isolated porcine coronary arteries precontracted with prostaglandin F2α (PGF2α; EC50=190 nM)[1]. MBCQ (0.01-10 μM; 10 min) inhibits Carbachol (10 μM)-induced contractions in a concentration-dependent manner in rat ileal smooth muscle[3]. |
| References |
| Molecular Formula | C16H12ClN3O2 |
|---|---|
| Molecular Weight | 313.73800 |
| Exact Mass | 313.06200 |
| PSA | 56.27000 |
| LogP | 3.69700 |
| RIDADR | NONH for all modes of transport |
|---|
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
BH3 domain-independent apolipoprotein L1 toxicity rescued by BCL2 prosurvival proteins.
Am. J. Physiol. Cell Physiol. 309 , C332-47, (2015) The potent trypanolytic properties of human apolipoprotein L1 (APOL1) can be neutralized by the trypanosome variant surface antigen gene product known as serum resistance-associated protein. However, ... |
| 4-((3,4-Methylenedioxybenzyl)amino)-6-chloroquinazoline |
| UPCMLD-DP008 |
| MBCQ |