Azilsartan
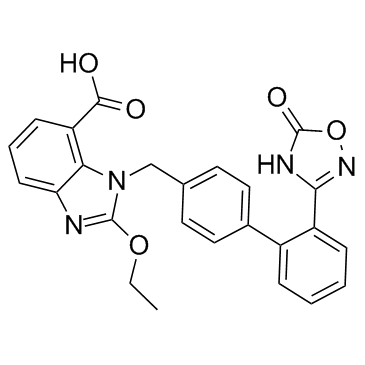
Azilsartan structure
|
Common Name | Azilsartan | ||
|---|---|---|---|---|
| CAS Number | 147403-03-0 | Molecular Weight | 456.450 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C25H20N4O5 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of AzilsartanAzilsartan(TAK-536) is a specific and potent angiotensin II type 1 receptor antagonist with IC50 of 2.6 nM.IC50 Value: 2.6 nM [1]Target: AT1 receptorin vitro: Azilsartan inhibited the specific binding of 125I-Sar1-Ile8-AII to human angiotensin type 1 receptors with an IC50 of 2.6 nM. The inhibitory effect of AZL persisted after washout of the free compound (IC(50) value of 7.4 nM). AZL also inhibited the accumulation of AII-induced inositol 1-phosphate (IP1) in the cell-based assay with an IC50 value of 9.2 nmol; this effect was resistant to washout (IC50 value of 81.3 nM). Olmesartan and valsartan inhibited IP1 accumulation with IC50 values of 12.2 and 59.8 nM, respectively [1]. Azilsartan is not readily biodegradable. Results of the water sediment study demonstrated significant shifting of azilsartan metabolites to sediment. Based on the equilibrium partitioning method, metabolites are unlikely to pose a risk to sediment-dwelling organisms [2].in vivo: In 4 randomized controlled trials (3 published to date), azilsartan medoxomil/chlorthalidone 40 mg/12.5 mg and 40 mg/25 mg reduced blood pressure (BP) significantly more than comparators did, including an approximately 5-mm Hg greater BP reduction than olmesartan medoxomil/hydrochlorothiazide 40 mg/25 mg and azilsartanmedoxomil/hydrochlorothiazide [3]. Both TAK-536 and candesartan suppressed the increase in plasma glucose level in the OGTT without significant change in insulin concentration and improved insulin sensitivity. In adipose tissue, TAK-536 and candesartan reduced TNF-alpha expression but increased the expression of adiponectin, PPARgamma, C/EBalpha, and aP2 [4].Clinical trial: New Angiotensin II Receptor Blocker Azilsartan Study for Stronger Blood Pressure Lowering . Phase4 |
| Name | azilsartan |
|---|---|
| Synonym | More Synonyms |
| Description | Azilsartan(TAK-536) is a specific and potent angiotensin II type 1 receptor antagonist with IC50 of 2.6 nM.IC50 Value: 2.6 nM [1]Target: AT1 receptorin vitro: Azilsartan inhibited the specific binding of 125I-Sar1-Ile8-AII to human angiotensin type 1 receptors with an IC50 of 2.6 nM. The inhibitory effect of AZL persisted after washout of the free compound (IC(50) value of 7.4 nM). AZL also inhibited the accumulation of AII-induced inositol 1-phosphate (IP1) in the cell-based assay with an IC50 value of 9.2 nmol; this effect was resistant to washout (IC50 value of 81.3 nM). Olmesartan and valsartan inhibited IP1 accumulation with IC50 values of 12.2 and 59.8 nM, respectively [1]. Azilsartan is not readily biodegradable. Results of the water sediment study demonstrated significant shifting of azilsartan metabolites to sediment. Based on the equilibrium partitioning method, metabolites are unlikely to pose a risk to sediment-dwelling organisms [2].in vivo: In 4 randomized controlled trials (3 published to date), azilsartan medoxomil/chlorthalidone 40 mg/12.5 mg and 40 mg/25 mg reduced blood pressure (BP) significantly more than comparators did, including an approximately 5-mm Hg greater BP reduction than olmesartan medoxomil/hydrochlorothiazide 40 mg/25 mg and azilsartanmedoxomil/hydrochlorothiazide [3]. Both TAK-536 and candesartan suppressed the increase in plasma glucose level in the OGTT without significant change in insulin concentration and improved insulin sensitivity. In adipose tissue, TAK-536 and candesartan reduced TNF-alpha expression but increased the expression of adiponectin, PPARgamma, C/EBalpha, and aP2 [4].Clinical trial: New Angiotensin II Receptor Blocker Azilsartan Study for Stronger Blood Pressure Lowering . Phase4 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C25H20N4O5 |
| Molecular Weight | 456.450 |
| Exact Mass | 456.143372 |
| PSA | 123.24000 |
| LogP | 4.21 |
| Appearance of Characters | white to beige |
| Index of Refraction | 1.695 |
| InChIKey | KGSXMPPBFPAXLY-UHFFFAOYSA-N |
| SMILES | CCOc1nc2cccc(C(=O)O)c2n1Cc1ccc(-c2ccccc2-c2noc(=O)[nH]2)cc1 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble15mg/mL (clear solution) |
| RIDADR | NONH for all modes of transport |
|---|
|
Liquid chromatography/tandem mass spectrometry study of forced degradation of azilsartan medoxomil potassium.
Rapid Commun. Mass Spectrom. 29 , 1437-47, (2015) Azilsartan medoxomil potassium (AZM) is a new antihypertensive drug introduced in the year 2011. The presence of degradation products not only affects the quality, but also the safety aspects of the d... |
|
|
Olmesartan blocks advanced glycation end products-induced vcam-1 gene expression in mesangial cells by restoring Angiotensin-converting enzyme 2 level.
Horm. Metab. Res. 46(6) , 379-83, (2014) Advanced glycation end products (AGEs) and their receptor (RAGE) system are involved in diabetic nephropathy. Angiotensin-converting enzyme 2 (ACE 2) plays a protective role against cardiovascular and... |
|
|
Azilsartan Improves Salt Sensitivity by Modulating the Proximal Tubular Na+-H+ Exchanger-3 in Mice.
PLoS ONE 11 , e0147786, (2016) A potent angiotensin II type-1 receptor blocker, azilsartan, has been reported to reduce blood pressure more effectively than candesartan. Interestingly, azilsartan can also restore the circadian rhyt... |
| TAK 536 |
| Azilsartan |
| 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-1H-benzimidazole-7-carboxylic acid |
| UNII-F9NUX55P23 |
| 1H-Benzimidazole-7-carboxylic acid,1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1'-biphenyl]-4-yl]methyl]-2-ethoxy |
| 2-ethoxy-3-[[4-[2-(5-oxo-2H-1,2,4-oxadiazol-3-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic acid |
| 1H-Benzimidazole-7-carboxylic acid, 1-[[2'-(4,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1'-biphenyl]-4-yl]methyl]-2-ethoxy- |
| 2-ethoxy-1-((2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-biphenyl-4-yl)methyl)-1H-benzimidazole-7-carboxylic acid |

