Pregnenolone 16α-carbonitrile
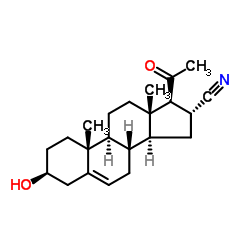
Pregnenolone 16α-carbonitrile structure
|
Common Name | Pregnenolone 16α-carbonitrile | ||
|---|---|---|---|---|
| CAS Number | 1434-54-4 | Molecular Weight | 341.487 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 508.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H31NO2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 261.3±30.1 °C | |
Use of Pregnenolone 16α-carbonitrilePregnenolone 16α-carbonitrile is an orally active prototypical and effective rodent-PXR activator. Pregnenolone 16α-carbonitrile, a synthetic steroid, induces cytochrome P4503A expression. Pregnenolone 16α-carbonitrile exhibits increased resistance to subsequent stressful insults[1][2][3]. |
| Name | pregnenolone 16α-carbonitrile |
|---|---|
| Synonym | More Synonyms |
| Description | Pregnenolone 16α-carbonitrile is an orally active prototypical and effective rodent-PXR activator. Pregnenolone 16α-carbonitrile, a synthetic steroid, induces cytochrome P4503A expression. Pregnenolone 16α-carbonitrile exhibits increased resistance to subsequent stressful insults[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Pregnenolone 16α-carbonitrile (20 μM; 9 days) directly inhibits rat hepatic stellate cell (HSCs) transdifferentiation to a profibrogenic phenotype, although the cells did not express the PXR (in contrast with hepatocytes)[4]. |
| In Vivo | Pregnenolone 16α-carbonitrile (40 mg/kg/day; i.p.; for two days) induces the expression of Cyp3a11 and Cyp2b10 at the mRNA, protein, and enzymatic levels in WT mice[1]. Pregnenolone 16α-carbonitrile (100 mg/kg; ip; single dose) induces the expression of CYP3A mRNA in adult female Sprague-Dawley rats weighing 150-200 g[2]. Pregnenolone 16α-carbonitrile (35 mg/kg; gavage; once daily for three days) increase in Pgp expression in male Sprague-Dawley rats, aged approximately 100 days and weighing 250-400 g[3]. Animal Model: WT and Pxr-/- mice[1] Dosage: 40 mg/kg Administration: IP; per day for two days Result: Induced the expression of Cyp3a11 and Cyp2b10 at the mRNA, protein, and enzymatic levels in WT mice. Had little effect on the expression of Cyp3a11 in Pxr-/- mice |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 508.5±50.0 °C at 760 mmHg |
| Molecular Formula | C22H31NO2 |
| Molecular Weight | 341.487 |
| Flash Point | 261.3±30.1 °C |
| Exact Mass | 341.235474 |
| PSA | 61.09000 |
| LogP | 3.22 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.558 |
| InChIKey | VSBHRRMYCDQLJF-ZDNYCOCVSA-N |
| SMILES | CC(=O)C1C(C#N)CC2C3CC=C4CC(O)CCC4(C)C3CCC21C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | TU4536500 |
|
~% 
Pregnenolone 16... CAS#:1434-54-4 |
| Literature: Tetrahedron, , vol. 7, p. 130,136 Journal of the Chemical Society, , p. 3748 Tetrahedron, , vol. 3, p. 37,40 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Pregnane X receptor agonists impair postprandial glucose tolerance.
Clin. Pharmacol. Ther. 93(6) , 556-63, (2013) We conducted a randomized, open, placebo-controlled crossover trial to investigate the effects of the pregnane X receptor (PXR) agonist rifampin on an oral glucose tolerance test (OGTT) in 12 healthy ... |
|
|
The cytochrome P450 2AA gene cluster in zebrafish (Danio rerio): expression of CYP2AA1 and CYP2AA2 and response to phenobarbital-type inducers.
Toxicol. Appl. Pharmacol. 272(1) , 172-9, (2013) The cytochrome P450 (CYP) 2 gene family is the largest and most diverse CYP gene family in vertebrates. In zebrafish, we have identified 10 genes in a new subfamily, CYP2AA, which does not show orthol... |
|
|
Decreased apoptosis during CAR-mediated hepatoprotection against lithocholic acid-induced liver injury in mice.
Toxicol. Lett. 188(1) , 38-44, (2009) Myeloid cell leukemia-1 (Mcl-1) is an anti-apoptotic protein that is regulated by the constitutive androstane receptor (CAR). Activation of CAR can protect the liver against bile acid-induced toxicity... |
| (3S,8S,9S,10R,13S,14S,16R,17S)-17-acetyl-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-16-carbonitrile |
| MFCD00079212 |
| (3β,16α)-3-Hydroxy-20-oxopregn-5-ene-16-carbonitrile |
| 16 α-Carbonitrile, Pregnenolone |
| 3beta-Hydroxy-20-oxo-5-pregnene-16alpha-carbonitrile |
| pregnenolone 16alpha-carbonitrile |
| pregnenolone carbonitrile |
| Pregn-5-ene-16-carbonitrile, 3-hydroxy-20-oxo-, (3β,16α)- |
| Pregnenolone 16α-carbonitrile |
| Pregnenolone-16alpha-carbonitrile |
| 5-Pregnen-3beta-ol-20-one-16alpha-carbonitrile |