4,6-O-Ethylidene-a-D-glucose
Modify Date: 2025-08-23 19:39:54
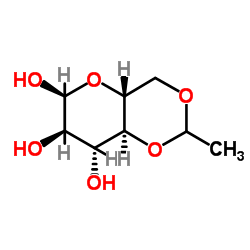
4,6-O-Ethylidene-a-D-glucose structure
|
Common Name | 4,6-O-Ethylidene-a-D-glucose | ||
|---|---|---|---|---|
| CAS Number | 13224-99-2 | Molecular Weight | 206.193 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 386.6±42.0 °C at 760 mmHg | |
| Molecular Formula | C8H14O6 | Melting Point | 168-170ºC | |
| MSDS | N/A | Flash Point | 187.6±27.9 °C | |
Use of 4,6-O-Ethylidene-a-D-glucose4,6-O-ethylidene-α-D-glucose (Ethylidene-glucose), a glucose derivative, is a competitive exofacial binding-site inhibitor on glucose transporter 1 (GLUT1) with a Ki of 12 mM for wild-type 2-deoxy-D-glucose transport[1][2][3]. |
| Name | 4,6-O-Ethylidene-α-D-glucose |
|---|---|
| Synonym | More Synonyms |
| Description | 4,6-O-ethylidene-α-D-glucose (Ethylidene-glucose), a glucose derivative, is a competitive exofacial binding-site inhibitor on glucose transporter 1 (GLUT1) with a Ki of 12 mM for wild-type 2-deoxy-D-glucose transport[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | 4,6-O-ethylidene-α-D-glucose (Ethylidene-glucose) shows poor affinity for malarial hexose transporter (PfHT1; Ki>50 mM). 4,6-O-ethylidene-α-D-glucose inhibits wild-type transport with a Ki of approximately 12 mM, but this is increased to greater than 120 mM in the Gln282-Leu mutant[1]. 4,6-O-ethylidene-α-D-glucose inhibits glucose exit competitively but its penetration into human red cells is unaffected by glucose in the medium. The potentiation of the development of FDNB inhibition by sugars in the incubating medium is absent when 4,6-O-ethylidene-α-D-glucose is used and there was a slight protective action. 4,6-O-ethylidene-α-D-glucose penetrates human red cells by simple diffusion supported by its penetration of guinea-pig red cells at similar rates, by the occurrence of osmotic haemolysis in isosmotic solutions which is unaffected by copper ions and by the relatively high ether/water partition of the compound<[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 386.6±42.0 °C at 760 mmHg |
| Melting Point | 168-170ºC |
| Molecular Formula | C8H14O6 |
| Molecular Weight | 206.193 |
| Flash Point | 187.6±27.9 °C |
| Exact Mass | 206.079041 |
| PSA | 88.38000 |
| LogP | 0.54 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.539 |
| WGK Germany | 3 |
|---|---|
| HS Code | 2932999099 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| MFCD00210951 |
| 4,6-O-Ethylidene-α-D-glucopyranose |
| α-D-Glucopyranose, 4,6-O-ethylidene- |
| 4,6-O-Ethylidene-a-D-glucose |
| 4,6-O-Ethylidene-α-D-glucose |
| (4aR,7R,8R,8aS)-2-Methylhexahydropyrano[3,2-d][1,3]dioxine-6,7,8-triol |
| (2R,4aR,6S,7R,8R,8aS)-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxine-6,7,8-triol |
| EINECS 236-496-2 |