AZD3514
Modify Date: 2025-08-25 16:13:02
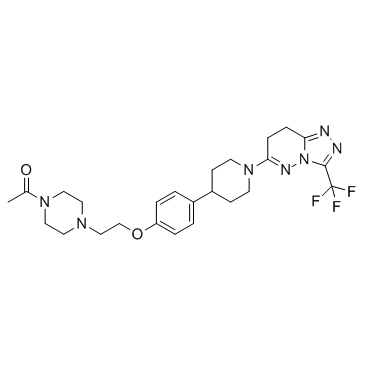
AZD3514 structure
|
Common Name | AZD3514 | ||
|---|---|---|---|---|
| CAS Number | 1240299-33-5 | Molecular Weight | 519.563 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 669.5±65.0 °C at 760 mmHg | |
| Molecular Formula | C25H32F3N7O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 358.7±34.3 °C | |
Use of AZD3514AZD3514 is a potent and oral androgen receptor downregulator with Ki of 2.2 μM and has ability of reducing AR protein expression.IC50 Value: 2.2 uM (Ki)Target: androgen receptorAZD3514 binds to the AR ligand binding domain and has selectivity for binding to AR over other nuclear hormone receptors [1]. in vitro: AZD3514 inhibits cell growth in prostate cancer cells expressing wild-type (VCaP) and mutated (T877A) AR (LNCaP), but is inactive in AR-negative prostate cancer cells, indicating a dependency on AR for efficacy [2]. in vivo: We assessed activity initially in the Hershberger castrated rat assay in which oral dosing of AZD3514 (100mg/kg once-daily for 7 days) significantly inhibited testosterone-induced growth of sexual accessory organs [2]. Clinical trial: Open-label Prostate Cancer Study. Phase 1 |
| Name | 1-[4-[2-[4-[1-[3-(trifluoromethyl)-7,8-dihydro-[1,2,4]triazolo[4,3-b]pyridazin-6-yl]piperidin-4-yl]phenoxy]ethyl]piperazin-1-yl]ethanone |
|---|---|
| Synonym | More Synonyms |
| Description | AZD3514 is a potent and oral androgen receptor downregulator with Ki of 2.2 μM and has ability of reducing AR protein expression.IC50 Value: 2.2 uM (Ki)Target: androgen receptorAZD3514 binds to the AR ligand binding domain and has selectivity for binding to AR over other nuclear hormone receptors [1]. in vitro: AZD3514 inhibits cell growth in prostate cancer cells expressing wild-type (VCaP) and mutated (T877A) AR (LNCaP), but is inactive in AR-negative prostate cancer cells, indicating a dependency on AR for efficacy [2]. in vivo: We assessed activity initially in the Hershberger castrated rat assay in which oral dosing of AZD3514 (100mg/kg once-daily for 7 days) significantly inhibited testosterone-induced growth of sexual accessory organs [2]. Clinical trial: Open-label Prostate Cancer Study. Phase 1 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 669.5±65.0 °C at 760 mmHg |
| Molecular Formula | C25H32F3N7O2 |
| Molecular Weight | 519.563 |
| Flash Point | 358.7±34.3 °C |
| Exact Mass | 519.256958 |
| PSA | 79.09000 |
| LogP | 1.16 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.642 |
| InChIKey | JMEYDSHPKCSIJC-UHFFFAOYSA-N |
| SMILES | CC(=O)N1CCN(CCOc2ccc(C3CCN(C4=Nn5c(nnc5C(F)(F)F)CC4)CC3)cc2)CC1 |
| Storage condition | -20℃ |
| S7040,AZD 3514 |
| 6-(4-{4-[2-(4-acetylpiperazin-1-yl)ethoxy]phenyl}piperidin-1-yl)-3-(trifluoromethyl)-7,8-dihydro[1,2,4]triazolo[4,3-b]pyridazine |
| 1-(4-(2-(4-(1-(3-(trifluoromethyl)-7,8-dihydro-[1,2,4]triazolo[4,3-b]pyridazin-6-yl)piperidin-4-yl)phenoxy)ethyl)piperazin-1-yl)ethanone |
| 1-{4-[2-(4-{1-[3-(Trifluoromethyl)-7,8-dihydro[1,2,4]triazolo[4,3-b]pyridazin-6-yl]-4-piperidinyl}phenoxy)ethyl]-1-piperazinyl}ethanone |
| Ethanone, 1-[4-[2-[4-[1-[7,8-dihydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-b]pyridazin-6-yl]-4-piperidinyl]phenoxy]ethyl]-1-piperazinyl]- |
| AZD3514 |