N6022
Modify Date: 2025-08-25 09:29:34
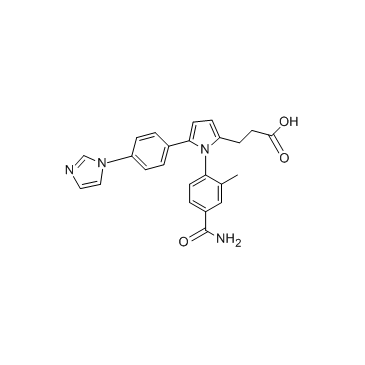
N6022 structure
|
Common Name | N6022 | ||
|---|---|---|---|---|
| CAS Number | 1208315-24-5 | Molecular Weight | 414.457 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 662.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C24H22N4O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 354.6±31.5 °C | |
Use of N6022N6022 is a potent, selective, reversible, and efficacious S-Nitrosoglutathione reductase(GSNOR) inhibitor with IC50 of 8 nM. |
| Name | 3-[1-(4-carbamoyl-2-methylphenyl)-5-(4-imidazol-1-ylphenyl)pyrrol-2-yl]propanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | N6022 is a potent, selective, reversible, and efficacious S-Nitrosoglutathione reductase(GSNOR) inhibitor with IC50 of 8 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 8 nM (GSNOR)[2] |
| In Vitro | N6022 shows concentration-dependent binding to rat plasma proteins. N6022 has more effect on ATP at lower drug concentrations (20 μM) than on GSH[1]. N6022 binds in the GSNO substrate binding pocket like a competitive inhibitor with an IC50 of 8 nM and a Ki of 2.5 nM. N6022 is uncompetitive with cofactors NAD+ and NADH[2]. |
| In Vivo | N6022 (50 mg/kg)-treated rats show a slight increase in the incidence of granulomas. In serum, N6022 remains in solution up to 5 mg/mL[1]. |
| Cell Assay | N6022 is tested using a rat hepatoma (H4IIE) cell line whereby cells are seeded into 96-well plates and cultured in medium containing 20% bovine serum. Following an equilibration period of 48 h, the cells are treated with N6022 (5% DMSO vehicle) at concentrations of 0, 1, 5, 10, 20, 50, 100, and 300 μM for 24 h at 37°C in 5% CO2. Camptothecin and rotenone are included as positive controls. The cell supernatant or the cells themselves are harvested for biochemical analysis. |
| References |
[4]. Thomas M. Raffay, et al. Methods of treating respiratory disorders. Patent. US 20170209419 A1. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 662.7±55.0 °C at 760 mmHg |
| Molecular Formula | C24H22N4O3 |
| Molecular Weight | 414.457 |
| Flash Point | 354.6±31.5 °C |
| Exact Mass | 414.169189 |
| PSA | 103.14000 |
| LogP | 3.35 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.664 |
| Storage condition | -20℃ |
| 3-{1-(4-Carbamoyl-2-methylphenyl)-5-[4-(1H-imidazol-1-yl)phenyl]-1H-pyrrol-2-yl}propanoic acid |
| 1H-Pyrrole-2-propanoic acid, 1-[4-(aminocarbonyl)-2-methylphenyl]-5-[4-(1H-imidazol-1-yl)phenyl]- |
| 3-(5-(4-(1H-Imidazol-1-yl)phenyl)-1-(4-carbamoyl-2-methylphenyl)-1H-pyrrol-2-yl)propanoic acid |
| N6022 |