aluminum boride
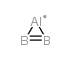
aluminum boride structure
|
Common Name | aluminum boride | ||
|---|---|---|---|---|
| CAS Number | 12041-50-8 | Molecular Weight | 50.61940 | |
| Density | 3.16 | Boiling Point | N/A | |
| Molecular Formula | AlB2H2 | Melting Point | decomposes to AlB12 at >920℃ [CIC73] | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Name | alumanylidyneborane |
|---|---|
| Synonym | More Synonyms |
| Density | 3.16 |
|---|---|
| Melting Point | decomposes to AlB12 at >920℃ [CIC73] |
| Molecular Formula | AlB2H2 |
| Molecular Weight | 50.61940 |
| Exact Mass | 51.01580 |
| Appearance of Characters | powder |
| InChIKey | DJPURDPSZFLWGC-UHFFFAOYSA-N |
| SMILES | B#[Al] |
| Water Solubility | soluble dilute HCl [CIC73] |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Examples of Hartmann-Hahn match conditions for CP/MAS between two half-integer quadrupolar nuclei.
J. Magn. Reson. 139(1) , 98-108, (1999) Hartmann-Hahn match conditions for n2 --> M2 CP/MAS between two quadrupolar nuclei, spin-lock signal as a function of effective nutation frequency, and the correlation of effective nutation frequency ... |
|
|
Chiral oxazaborolidine-aluminum bromide complexes are unusually powerful and effective catalysts for enantioselective Diels-Alder reactions.
J. Am. Chem. Soc. 129(6) , 1498-9, (2007)
|
|
|
Fabrication and characterization of aluminum nitride/boron nitride nanocomposites by carbothermal reduction and nitridation of aluminum borate powders.
J. Nanosci. Nanotechnol. 8(11) , 5846-53, (2008) In order to fabricate aluminum nitride/boron nitride (AIN/BN) nanocomposites by pressureless sintering, the present study investigated the synthesis of AIN-BN nanocomposite powders by carbothermal red... |
| Aluminum diboride -325 mesh |
| MFCD00084760 |
| ALUMINUM BORIDE |
| EINECS 234-923-7 |

