Endoxifen (E-isomer hydrochloride)
Modify Date: 2024-01-28 17:04:23
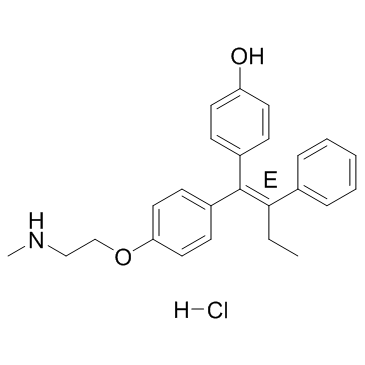
Endoxifen (E-isomer hydrochloride) structure
|
Common Name | Endoxifen (E-isomer hydrochloride) | ||
|---|---|---|---|---|
| CAS Number | 1197194-61-8 | Molecular Weight | 409.948 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C25H28ClNO2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Endoxifen (E-isomer hydrochloride)Endoxifen (E-isomer hydrochloride) is a tamoxifen metabolite and potent Selective Estrogen Response Modifier (SERM). Target: Estrogen Receptor/ERREndoxifen is considered a prodrug, since it has a much higher potency for the estrogen receptor than its parent drug. Endoxifen inhibits the hERG channel protein trafficking to the plasma membrane in a concentration-dependent manner with Endoxifen being more potent than Tamoxifen. [1] Endoxifen is also shown to be a more potent inhibitor of estrogen target genes when ERβ is expressed. Additionally, low concentrations of Endoxifen observed in Tamoxifen treated patients with deficient CYP2D6 activity (20 to 40 nM) markedly inhibit estrogen-induced cell proliferation rates in the presence of ERβ, whereas much higher Endoxifen concentrations are needed when ERβ is absent.[2] |
| Name | 4-[(1E)-1-{4-[2-(Methylamino)ethoxy]phenyl}-2-phenyl-1-buten-1-yl]phenol hydrochloride (1:1) |
|---|---|
| Synonym | More Synonyms |
| Description | Endoxifen (E-isomer hydrochloride) is a tamoxifen metabolite and potent Selective Estrogen Response Modifier (SERM). Target: Estrogen Receptor/ERREndoxifen is considered a prodrug, since it has a much higher potency for the estrogen receptor than its parent drug. Endoxifen inhibits the hERG channel protein trafficking to the plasma membrane in a concentration-dependent manner with Endoxifen being more potent than Tamoxifen. [1] Endoxifen is also shown to be a more potent inhibitor of estrogen target genes when ERβ is expressed. Additionally, low concentrations of Endoxifen observed in Tamoxifen treated patients with deficient CYP2D6 activity (20 to 40 nM) markedly inhibit estrogen-induced cell proliferation rates in the presence of ERβ, whereas much higher Endoxifen concentrations are needed when ERβ is absent.[2] |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C25H28ClNO2 |
|---|---|
| Molecular Weight | 409.948 |
| Exact Mass | 409.180847 |
| Storage condition | 2-8℃ |
| 4-[(1E)-1-{4-[2-(Methylamino)ethoxy]phenyl}-2-phenyl-1-buten-1-yl]phenol hydrochloride (1:1) |
| Phenol, 4-[(1E)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]-, hydrochloride (1:1) |
| Endoxifen |
| E-isomer hydrochloride |
| Endoxifen (E-isomer hydrochloride) |