| Description |
Urolithin A is an intestinal metabolite of ellagic acid with antioxidant and antiproliferative effects; inhibits T24 and Caco-2 cell growth with IC50 values of 43.9 and 49 μM, respectively.
|
| Related Catalog |
|
| Target |
IC50: 43.9 μM (T24 cell)[1], 49 μM (Caco-2cell)[2]
|
| In Vitro |
Urolithins could mainly inhibit prostate cancer and colon cancer cell growth. Urolithin A increases mRNA and protein expression of Phospho-p38 MAPK, and decreases mRNA and protein expression of MEKK1 and Phospho-c-Jun in T24 cells. Caspase-3 is also activated and PPAR-γ protein expression increased in drug-induced apoptosis[1]. Urolithin A exerts a dose- and time-dependent significant arrest at G2/M and S phases after treatments with 50 and 100 μM at 24 and 48 h compared to control cells. It induces cell apoptosis with 50 and 100 μM [2]. Urolithin A shows potent antiproliferative activity on HepG2 cells. When cell death is induced by Urolithin A, the expression of β-catenin, c-Myc and Cyclin D1 are decreased and TCF/LEF transcriptional activation is notably down-regulated. Urolithin A also increases protein expression of p53, p38-MAPK and caspase-3, but suppresses expression of NF-κB p65 and other inflammatory mediators[3].
|
| In Vivo |
The volume of paw edema is reduced at 1 h after oral administration of urolithin A. In addition, plasma in treated mice exhibited significant oxygen radical antioxidant capacity (ORAC) scores with high plasma levels of the unconjugated form at 1 h after oral administration of urolithin A[4].
|
| Cell Assay |
Human colon cancer cells HT-29 are treated for 24 and 48 h at 100 and 50 μM of Urolithin A and Iso Urolithin A aglycones and their glucuronide conjugates. Cell viability and proliferation are measured using a TC10 automated cell counter with the addition of Trypan blue for viability determination. IC50 values are determined by MTT assay[2].
|
| Animal Admin |
Mice: Paw edema is induced in the right hind paw of ICR mice by the subcutaneous injection of 1% λ-carrageenan in pysiological saline (50 μL). The inflammation level is quantified by the volume of paw edema. Urolithin A dissolved in 0.5% carboxymethylcellulose suspension is orally administered to the mice at 1 or 6 h before carrageenan injection. The anti-inflammatory effects of urolithin A on carrageenan-induced edema in mice are analyzed[4].
|
| References |
[1]. Qiu Z, et al. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem Toxicol. 2013 Sep;59:428-37. [2]. González-Sarrías A, et al. Antiproliferative activity of the ellagic acid-derived gut microbiota isourolithin A and comparison with its urolithin A isomer: the role of cell metabolism.Eur J Nutr. 2017 Mar;56(2):831-841. [3]. Wang Y, et al. In vitro antiproliferative and antioxidant effects of urolithin A, the colonic metabolite of ellagic acid, on hepatocellular carcinomas HepG2 cells. Toxicol In Vitro. 2015 Aug;29(5):1107-15. [4]. Ishimoto H, et al. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg Med Chem Lett. 2011 Oct 1;21(19):5901-4.
|
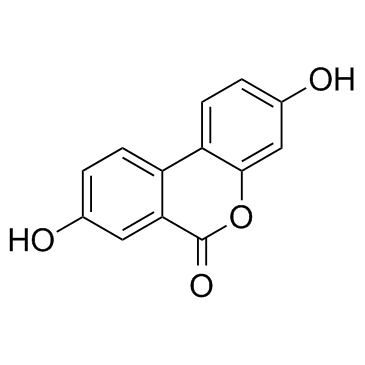
 CAS#:35233-17-1
CAS#:35233-17-1 CAS#:58380-11-3
CAS#:58380-11-3 CAS#:108-46-3
CAS#:108-46-3 CAS#:62924-93-0
CAS#:62924-93-0 CAS#:133730-33-3
CAS#:133730-33-3 CAS#:10417-94-4
CAS#:10417-94-4 CAS#:6217-54-5
CAS#:6217-54-5 CAS#:6971-51-3
CAS#:6971-51-3 CAS#:586-38-9
CAS#:586-38-9