Dolutegravir Sodium
Modify Date: 2025-08-25 08:18:47
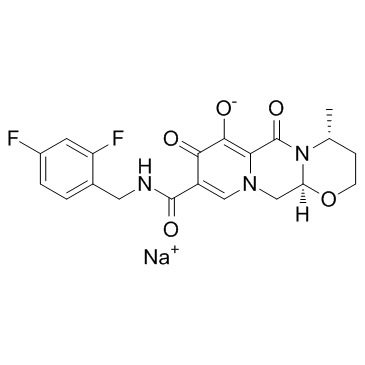
Dolutegravir Sodium structure
|
Common Name | Dolutegravir Sodium | ||
|---|---|---|---|---|
| CAS Number | 1051375-19-9 | Molecular Weight | 441.361 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C20H18F2N3NaO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Dolutegravir SodiumDolutegravir sodium is an inhibitor of HIV-1 integrase-catalyzed strand transfer with IC50 of 2.7 nM. |
| Name | dolutegravir sodium |
|---|---|
| Synonym | More Synonyms |
| Description | Dolutegravir sodium is an inhibitor of HIV-1 integrase-catalyzed strand transfer with IC50 of 2.7 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.7 nM (HIV-1 integrase)[1] |
| In Vitro | The EC50 of Dolutegravir (S/GSK1349572) against HIV-1 is 0.51 nM in PBMCs, 0.71 nM in MT-4 cells, and 2.2 nM in the PHIV assay, which uses a pseudotyped self-inactivating virus. The 50% cytotoxic concentrations (CC50) for Dolutegravir in proliferating IM-9, U-937, MT-4, and Molt-4 cells are 4.8, 7.0, 14, and 15 μM, respectively. In unstimulated and stimulated PBMCs, the CC50 are 189 μM and 52 μM, respectively. Based on the EC50 of Dolutegravir against HIV-1 in PBMCs (i.e., 0.51 nM), this translates to a cell-based therapeutic index of at least 9,400[1]. |
| In Vivo | Following a single intravenous (IV) administration of Dolutegravir, the plasma clearance is low in rats (0.23 mL/min/kg) and monkeys (2.12 mL/min/kg). The half-lives in the rat and monkey are similar, approximately 6 h, and the steady-state volume of distribution (VSS) is low. Following oral administration, Dolutegravir is rapidly absorbed with a high oral bioavailability when administered as a solution to fasted male rats and a single monkey (75.6 and 87.0%, respectively). Dolutegravir exposure (Cmax and AUC) increased with increasing dose following oral administration of a suspension to non-fasted rats up to 250 mg/kg and non-fasted monkeys up to 50 mg/kg, although the increase is less than proportional[2]. |
| Cell Assay | In vitro growth inhibition (cytotoxicity) studies are conducted with S/GSK1349572 (0.16, 0.8, 4, and 20 nM) in proliferating human leukemic and lymphomic cell lines (IM-9, U-937, MT-4, and Molt-4) as well as in stimulated and unstimulated human PBMCs. ATP levels are quantified by using the CellTiter-Glo luciferase reagent to measure the ability of a compound to inhibit cell growth as an indicator of the compound's potential for cytotoxicity[1]. |
| Animal Admin | For rat and monkey PK studies, Dolutegravir is administered as the free acid or the sodium salt. All doses are presented in terms of the free acid. Dolutegravir is administered by intravenous (IV) short-term (within 2 min) bolus (1 mg/kg) to three male rats and two male monkeys. For single oral administration, Dolutegravir as a solution (5 mg/kg) is administered to three fasted male rats and two fasted male monkeys. Dolutegravir is administered as single oral doses of 5, 50, 100, and 250 mg/kg to non-fasted male rats (n=2/dose level) and 3, 10, and 50 mg/kg to non-fasted female monkeys. For intravenous administration, blood samples are collected from rats (0.2 mL via jugular vein cannula) and monkeys (approximately 0.2 or 0.5 mL via saphenous vein in a hindlimb) into Na2EDTA-treated syringes at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 24 h. For oral administration, samples are collected at 0.25 (rats only), 0.5, 1, 2, 4, 6 [rats (solution and suspension) and monkey (solution only)], 8, and 24 h. Following collection, the blood is immediately put on wet ice and then centrifuged within an hour at 1740 g for 10 min at 4°C to obtain plasma. All samples are stored at approximately -20°C or colder prior to analysis by using a method based on protein precipitation and LC-MS/MS analysis. |
| References |
| Molecular Formula | C20H18F2N3NaO5 |
|---|---|
| Molecular Weight | 441.361 |
| Exact Mass | 441.111237 |
| PSA | 107.19000 |
| LogP | 2.30370 |
| Storage condition | -20℃ |
| 2H-Pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide, N-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-, sodium salt, (4R,12aS)- (1:1) |
| GSK1349572 sodiuM salt |
| sodium,(4R,12aS)-9-[(2,4-difluorophenyl)methylcarbamoyl]-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2H-pyrido[5,6]pyrazino[2,6-b][1,3]oxazin-7-olate |
| (4R,12aS)-N-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino-[2,1-b][1,3]oxazine-9-carboxamide sodium salt |
| UNII:1Q1V9V5WYQ |
| Sodium (4R,12aS)-9-[(2,4-difluorobenzyl)carbamoyl]-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazin-7-olate |
| Dolutegravir sodium |
| Sodium (4R,12aS)-9-{[(2,4-difluorophenyl)methyl]carbamoyl}-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazin-7-olate |
| Tivicay |
| sodium (4R,12aS)-9-((2,4-difluorobenzyl)carbamoyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2',4,5]pyrazino[2,1-b][1,3]oxazin-7-olate |
| (4R,12aS)-N-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide, sodium salt (1:1) |
| Dolutegravir (sodium) |

