6724-53-4
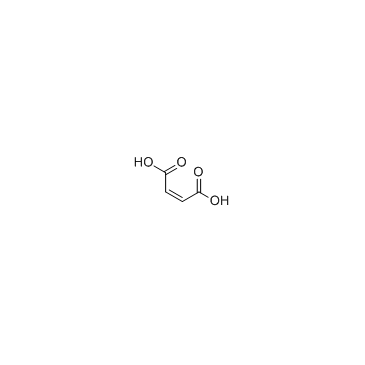
| Name | (Z)-but-2-enedioic acid,2-(2,2-dicyclohexylethyl)piperidine |
|---|---|
| Synonyms |
perhexilline maleate
Perhexiline maleate (USAN) EINECS 229-775-5 Pexid 2-(2,2-Dicyclohexylethyl)piperidine maleate DSSTox_CID_1114 perhexilene maleate Perhexiline maleate |
| Description | Perhexiline maleate is a potent carnitine palmitoyltransferase 1 (CPT 1) inhibitor with IC50s of 77 and 148 μM for rat heart and liver CPT 1, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 77 μM (Rat heart CPT 1), 148 μM (Rat liver CPT 1)[1] |
| In Vitro | At 24, 48 and 72 h of treatment with 0.01 and 1 μM of Perhexiline , NDM29 ncRNA expression level in SH-SY5Y cells is progressively increased, reaching a peak after 48 hours of treatment. Perhexiline treatment increases the susceptibility of NB cells to antiblastic treatments. Co-administration of Perhexiline maleate potentiates the efficacy of cisplatin to reduce the in vitro clonogenic potential of NB cells[2]. |
| In Vivo | The co-administration of Perhexiline and cisplatin yields a clear enhancement of antitumor effects, resulting in a significantly improved progression-free survival as compared with mice treated with DMSO. Perhexiline favors NB cell transition to differentiated phenotype[2]. |
| Cell Assay | The effect of antitumoral drugs on neuroblastoma cell survival is evaluated using the MTT assay. Approximately 24 hours after plating, cells are exposed to Perhexiline maleate (0.01 μM) for 48 hours at 37°C. Cytotoxicity is expressed as the percentage of cells surviving in relation to untreated cells[2]. |
| Animal Admin | Mice: In protocol a, 21 mice are divided in 4 groups: control vehicle group: DMSO; cisplatin (3 mg/kg/dose) treated group; Perhexiline (1 mg/kg/dose) treated group and; Perhexiline (1 mg/kg/dose) and cisplatin (3 mg/kg/dose) treated group. In protocol b, 20 mice are divided in 4 groups: control vehicle group: DMSO; Perhexiline (3 mg/kg/dose) treated group; cisplatin (5 mg/kg/dose) treated group; Perhexiline (3 mg/kg/dose) and cisplatin (5 mg/kg/dose) treated group[2]. |
| References |
| Boiling Point | 574.1ºC at 760 mmHg |
|---|---|
| Melting Point | 181-183ºC |
| Molecular Formula | C23H39NO4 |
| Molecular Weight | 393.56000 |
| Flash Point | 301ºC |
| Exact Mass | 393.28800 |
| PSA | 86.63000 |
| LogP | 5.33600 |
| Appearance | white to tan |
| Storage condition | -20?C Freezer, Under Inert Atmosphere |
| Water Solubility | DMSO: ≥5mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| WGK Germany | 2 |
| RTECS | TM7068000 |
| HS Code | 2933399090 |
|
~% 
6724-53-4 |
| Literature: US4191828 A1, ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |