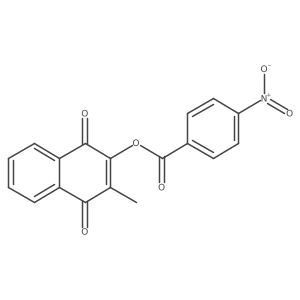
14748-94-8
| Name | (1,4-dihydroxy-3-methylnaphthalen-2-yl) 4-aminobenzoate |
|---|---|
| Synonyms |
1,4-Dihydroxy-3-methyl-2-naphthyl 4-aminobenzoate
UNII-03JLX11PE9 Capilarema Aminaftone Aminaphtone |
| Description | Aminaftone, a derivative of 4-aminobenzoic acid, downregulates endothelin-1 (ET-1) production in vitro by interfering with the transcription of the pre-pro-ET-1 gene. |
|---|---|
| Related Catalog | |
| Target |
endothelin-1 (ET-1)[1] |
| In Vitro | Aminaftone inhibits Endothelin-1 (ET-1) production in cell cultures by interfering with transcription of the pre-pro-endothelin-1 (PPET-1) gene. Incubation with IL-1beta increases concentrations of ET-1 and PPET-1 relative gene expression. Incubation with Aminaftone significantly reduces production of ET-1 in a concentration-dependent manner. A strong direct correlation is found between ET-1 concentrations and PPET-1 relative gene expression, but Aminaftone does not influence endothelin-converting enzyme (ECE) activity[1]. |
| In Vivo | The rats are randomly assigned to the following experimental groups: Control; Monocrotaline; Aminaftone 30 mg/kg/day; Aminaftone 150 mg/kg/day. After 5 weeks, mortality is significantly lower in the animals treated with Aminaftone at both doses compared to Monocrotaline alone. Aminaftone reduces plasma concentration of ET-1 and seems to reduce right heart hypertrophy and the wall thickness of the pulmonary arteries at the highest dose. At the end of the experiment, no rats die in the control and Aminaftone 150 groups, while mortality is 38% in the Monocrotaline group and 13% in the Aminaftone 30 group. Overall, Aminaftone treated rats have a significantly lower mortality compared to rats in the Monocrotaline group (P=0.044)[2]. |
| Cell Assay | Human ECV304 endothelial cells are incubated with interleukin-1beta (IL-1beta) 100 IU/mL with or without the addition of increasing concentrations of Aminaftone (2, 4 or 6 μg/mL). ET-1 concentrations in surnatants are quantified by enzyme immunoassay kit at 3, 6 and 12 hours. PPET-1 gene expressions are also analysed by real-time polymerase chain reaction (RT-PCR) at the same time points. ECE activity is also determined[1]. |
| Animal Admin | Rats[2] Male Wistar rats weighing 300±30 g are randomly assigned to one of the following four experimental groups: 1) control healthy rats (n=11); 2) only injection of Monocrotaline (n=12); 3) injection of Monocrotaline followed by Aminaftone 30 mg/kg treatment (Aminaftone30) (n=8); and 4) injection of Monocrotaline followed by Aminaftone 150 mg/kg treatment (Aminaftone150) (n=8). Aminaftone is administered as food admix. Rats are weighed twice a week. |
| References |
| Density | 1.39g/cm3 |
|---|---|
| Boiling Point | 617.1ºC at 760mmHg |
| Molecular Formula | C18H15NO4 |
| Molecular Weight | 309.31600 |
| Flash Point | 327ºC |
| Exact Mass | 309.10000 |
| PSA | 92.78000 |
| LogP | 3.94200 |
| Vapour Pressure | 8.03E-16mmHg at 25°C |
| Index of Refraction | 1.727 |
| Storage condition | 2-8℃ |
|
~% 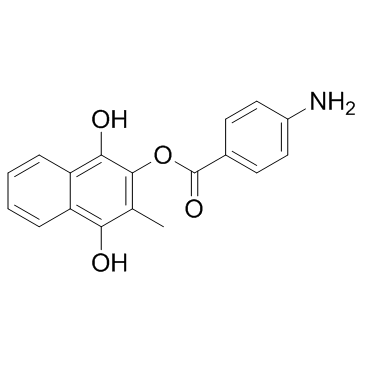
14748-94-8 |
| Literature: Laboratori Baldacci S.P.A. Patent: EP2390246 A1, 2011 ; Location in patent: Page/Page column 5 ; |