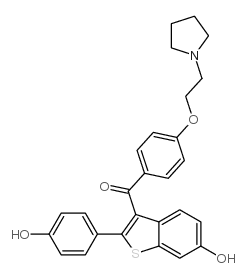
63676-22-2
| Name | 2-(4-hydroxyphenyl)-1-benzothiophen-6-ol |
|---|---|
| Synonyms |
2-(4-hydroxyphenyl)-6-hydroxybenzo[b]thiophene
2-(4-Hydroxyphenyl)benzo[b]thiophen-6-ol 2-(4-Hydroxyphenyl)benzo[b]thiophene-6-ol 6-hydroxy-2-(4-hydroxyphenyl)benzo<b>thiophene ZTW RALOXIFENE CORE benzthiophene compound,3 |
| Description | Estrogen receptor modulator 1 (compound 18) is an orally active and selective estrogen receptor modulator (SERM), with a pIC50 of 0.46. Estrogen receptor modulator 1 induces regression of Tamoxifen-resistant, hormone independent xenograft tumors[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Estrogen receptor modulator 1 (compound 18) (100 nM; 10 days) inhibits T47D:A18/PKCα and T47D:A18-TAM1 colony formation[2]. Estrogen receptor modulator 1 (100 nM; 9 days) significantly inhibits the growth of MCF-7:5C cell, and induces apoptosis in these cells 6 days[2]. Cell Proliferation Assay[2] Cell Line: T47D:A18/PKCα and T47D:A18-TAM1 cells Concentration: 100 nM Incubation Time: 10 days Result: Inhibit T47D:A18/PKCα and T47D:A18-TAM1 colony formation. Cell Viability Assay[2] Cell Line: MCF-7:5C cells Concentration: 100 nM Incubation Time: 9 days Result: Significantly inhibited the growth of MCF-7:5C cells. |
| In Vivo | Estrogen receptor modulator 1 (1.5 mg/animal; p.o. ; daily for 2 weeks) results in regression of T47D:A18/PKCα tumors[2]. Animal Model: 4-6 week old athymic mice (Harlan-Sprague-Dawley)[2] Dosage: 1.5 mg/animal Administration: p.o. ; daily for 2 weeks Result: Significantly reduced tumor volume. |
| References |
| Density | 1.383 |
|---|---|
| Boiling Point | 477ºC at 760 mmHg |
| Melting Point | 295 °C(dec.) |
| Molecular Formula | C14H10O2S |
| Molecular Weight | 242.29300 |
| Flash Point | 242.3ºC |
| Exact Mass | 242.04000 |
| PSA | 68.70000 |
| LogP | 3.97950 |
| Index of Refraction | 1.742 |
|
~71% 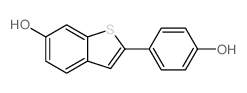
63676-22-2 |
| Literature: Jones, Charles D.; Jevnikar, Mary G.; Pike, Andrew J.; Peters, Mary K.; Black, Larry J.; at al. Journal of Medicinal Chemistry, 1984 , vol. 27, # 8 p. 1057 - 1066 |
|
~% 
63676-22-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 45, # 7 p. 1399 - 1401 |
|
~% 
63676-22-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 27, # 8 p. 1057 - 1066 |
|
~% 
63676-22-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 45, # 7 p. 1399 - 1401 |
|
~% 
63676-22-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 45, # 7 p. 1399 - 1401 |
|
~% 
63676-22-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 27, # 8 p. 1057 - 1066 |
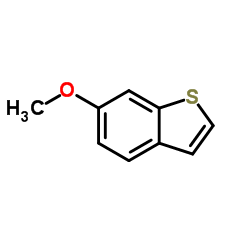
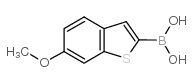
| Precursor 6 | |
|---|---|
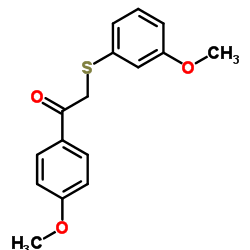
| DownStream 5 | |


![[4-(6-acetyloxy-1-benzothiophen-2-yl)phenyl] acetate structure](https://image.chemsrc.com/caspic/336/84449-63-8.png)
![4-(6-(BENZOYLOXY)BENZO[B]THIOPHEN-2-YL)PHENYL BENZOATE structure](https://image.chemsrc.com/caspic/298/84449-64-9.png)
![4-(6-((METHYLSULFONYL)OXY)BENZO[B]THIOPHEN-2-YL)PHENYL METHANESULFONATE structure](https://image.chemsrc.com/caspic/250/84449-65-0.png)