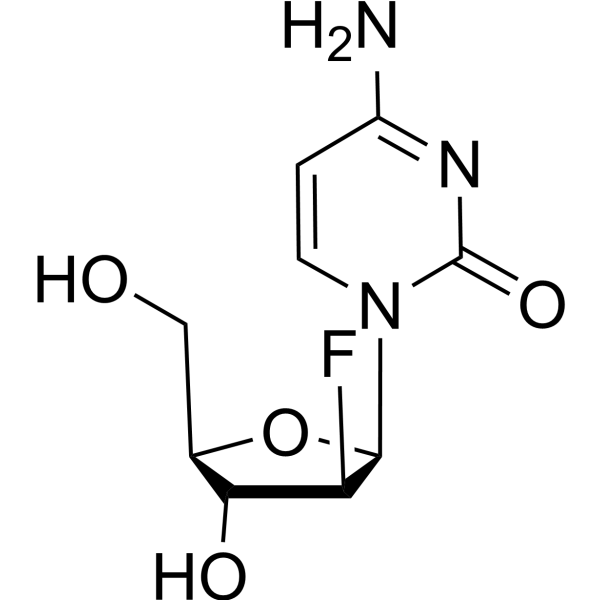
69123-90-6
| Name | 4-amino-1-[(2R,3S,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidin-2-one |
|---|---|
| Synonyms |
1-(2-Deoxy-2-fluoro-b-D-arabinofuranosyl)-5-iodocytosine
2'-Fluoro-5-iodo-1-b-D-arabinofuranosylcytosine 4-Amino-1-(2-deoxy-2-fluoro-b-D-arabinofuranosyl)-5-iodo-2(1H)-pyrimidinone FIAC 2'-Fluoro-5-iodo-aracytosine 2'-fluoro-5-iodoarabinosylcytosine Fluorviodoaracytidine DRG-0077 4-Amino-1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodo-2(1H)-pyrimidinone 4-Amino-1-(2-deoxy-2-fluor-β-D-arabinofuranosyl)-5-iodpyrimidin-2(1H)-on 4-Amino-1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodopyrimidin-2(1H)-one Fluoroiodoaracytidine Fiacitabine |
| Description | Fiacitabine(NSC 382097; FIAC; FOAC) is a selective inhibitior of DNA replication of herpes simplex virus(HSV) with IC50 values of 2.5 nM and 12.6 nM for HSV1 and HSV2, respectively. IC50 value: 2.5/12.6 nM (HSV1/2) [2]Target: HSVFIAC suppressed by 90% the replication of various strains of herpes simplex virus types 1 and 2 at concentrations of 0.0025 to 0.0126 microM. Cytotoxicity was minimal, as determined by trypan blue dye exclusion with norman Vero, WI-38, and NC-37 cell proliferation; the 50% inhibitory dose was 4 to 10 microM in a 4-day assay. FIAC was active at much lower concentrations than arabinosylcytosine, iododeoxyuridine, and arabinosyladenine. It was slightly more active against herpes simplex virus type 1 than acycloquanosine and slightly more toxic to normal cells. FIAC was about 8,000 times more active against the replication of wild-type herpes simplex virus type 1 than against a mutant strain lacking the expression of virus-specified thymidine kinase [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 2.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 524.6±60.0 °C at 760 mmHg |
| Molecular Formula | C9H11FIN3O4 |
| Molecular Weight | 371.104 |
| Flash Point | 271.1±32.9 °C |
| Exact Mass | 370.977814 |
| PSA | 110.60000 |
| LogP | -0.27 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.791 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
|
~% 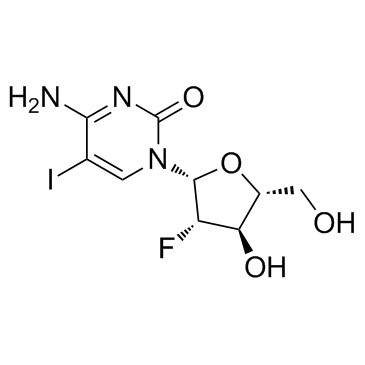
69123-90-6 |
| Literature: Tetrahedron, , vol. 68, # 26 p. 5145 - 5151 |
|
~% 
69123-90-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 26, # 2 p. 152 - 156 |
|
~% 
69123-90-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 26, # 2 p. 152 - 156 |
|
~% 
69123-90-6 |
| Literature: Tetrahedron, , vol. 68, # 26 p. 5145 - 5151 |
|
~% 
69123-90-6 |
| Literature: Tetrahedron, , vol. 68, # 26 p. 5145 - 5151 |
|
~% 
69123-90-6 |
| Literature: Tetrahedron, , vol. 68, # 26 p. 5145 - 5151 |
|
~% 
69123-90-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 26, # 2 p. 152 - 156 |
| Precursor 7 | |
|---|---|
| DownStream 3 | |
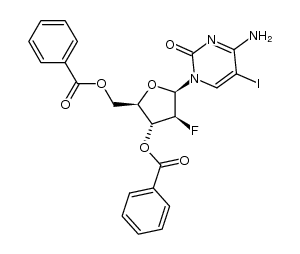
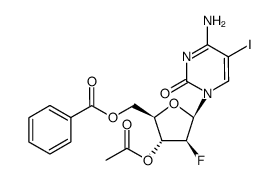
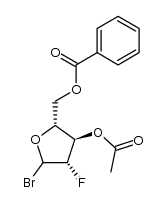
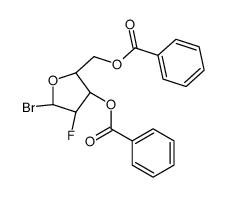
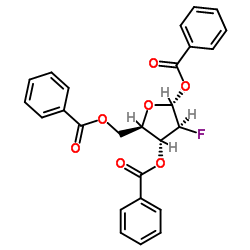
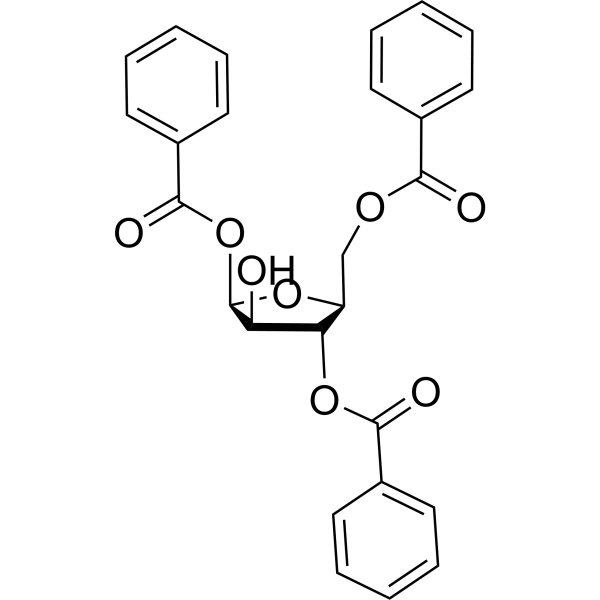
![4-amino-1-[(2R,3S,4R,5R)-3-fluoro-4-hydroxy-5-methyloxolan-2-yl]pyrimidin-2-one structure](https://image.chemsrc.com/caspic/474/105281-07-0.png)
![4-amino-1-[(2R,3S,4R,5R)-3-fluoro-4-hydroxy-5-methyloxolan-2-yl]-5-iodopyrimidin-2-one structure](https://image.chemsrc.com/caspic/479/105309-31-7.png)