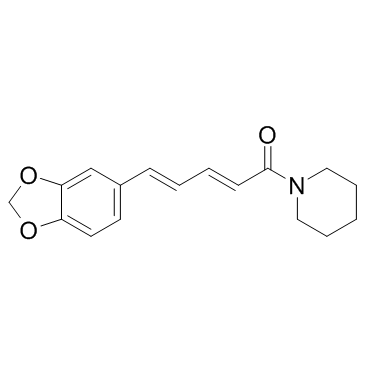
23434-88-0
| Name | 5-(1,3-benzodioxol-5-yl)-1-piperidin-1-ylpentan-1-one |
|---|---|
| Synonyms |
1-[5-(1,3-Benzodioxol-5-yl)pentanoyl]piperidine
Tetrahydropiperidine 5-(1,3-Benzodioxol-5-yl)-1-(piperidin-1-yl)pentan-1-one Tetrahydropiperin 5-(1,3-Benzodioxol-5-yl)-1-(1-piperidinyl)-1-pentanone Cosmoperine Tetrahydropiperine |
| Description | Tetrahydropiperine, a cyclohexyl analogue of piperine, is the first natural aryl pentanamide from Piper longum[1]. Tetrahydropiperine (compound 14) inhibits the cytochrome P450 (CYP) isoform CYP1A1/arylhydrocarbon hydroxylase (AHH; IC50=23 µM)[2]. |
|---|---|
| Related Catalog | |
| Target |
CYP1A1:23 μM (IC50) |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 469.9±24.0 °C at 760 mmHg |
| Melting Point | 41ºC |
| Molecular Formula | C17H23NO3 |
| Molecular Weight | 289.369 |
| Flash Point | 238.0±22.9 °C |
| Exact Mass | 289.167786 |
| PSA | 38.77000 |
| LogP | 3.67 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.556 |
| Storage condition | -20℃ |
|
~96% 
23434-88-0 |
| Literature: Sondengam, B. Lucas; Fomum, Z. Tanee; Charles, Georges; Akam, T. Mac Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1983 , p. 1219 - 1222 |
|
~% 
23434-88-0 |
| Literature: Kiuchi, Fumiyuki; Nakamura, Norio; Saito, Makiko; Komagome, Kazue; Hiramatsu, Hirokuni; Takimoto, Noriaki; Akao, Nobuaki; Kondo, Kaoru; Tsuda, Yoshisuke Chemical and Pharmaceutical Bulletin, 1997 , vol. 45, # 4 p. 685 - 696 |