166335-18-8
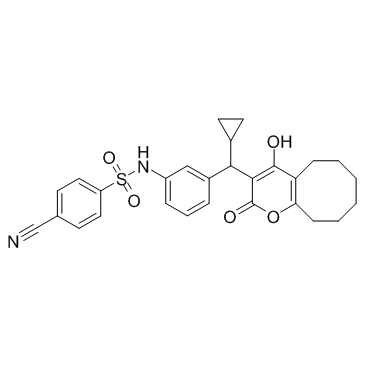
| Name | 4-cyano-N-[3-[cyclopropyl-(4-hydroxy-2-oxo-5,6,7,8,9,10-hexahydrocycloocta[b]pyran-3-yl)methyl]phenyl]benzenesulfonamide |
|---|---|
| Synonyms |
4-cyano-N-[3-[cyclopropyl(5,6,7,8,9,10-hexahydro-4-hydroxy-2-oxo-2H-cycloocta[b]pyran-3-yl)methyl]phenyl]-benzenesulfonamide
4-cyano-n-{3-[cyclopropyl(2-hydroxy-4-oxo-5,6,7,8,9,10-hexahydro-4h-cycloocta[b]pyran-3-yl)methyl]phenyl}benzenesulfonamide PNU-103017 |
| Description | PNU-103017 is an HIV protease inhibitor. |
|---|---|
| Related Catalog | |
| Target |
HIV protease[1] |
| In Vivo | PNU-103017 is a selective HIV aspartyl protease inhibitor under evaluation as a potential oral treatment of Acquired Immunodeficiency Diseases. PNU-103017 is a racemic mixture of two enantiomers, designated PNU-103264 (R-) and PNU-103265 (S-). The Cmax (P≤0.0349), Cmin (P≤0.0168), and Cav (P≤0.0118) are significantly higher for the (R)- than the (S)-enantiomer, showing enantioselective pharmacokinetics of PNU-103017 in the dog[1]. |
| Animal Admin | Rats and dogs are used in this study. In preclinical toxicology studies, dogs (three per gender per dose) receive 50, 100, 200, or 250 mg/kg/day, and rats (three per gender per dose) receive 80, 240, or 720 mg/kg/day PNU-103017 in a 0.5 N sodium hydroxide aqueous solution orally, twice daily, 8 and 16 h apart, respectively, for 14 days. Sequential blood specimens are collected at 0 (prior to the first daily dosing), 1, 2, 4, 8 (prior to the second daily dosing), 9, 10, 12, 16 (for dog only), and 24 h after the first daily dosing on treatment days 1, 8, and 14 for the dog and rat studies. The samples are placed into heparinized tubes, and the plasma is separated by centrifugation and stored at or below -10°C until analysis[1]. |
| References |
| Density | 1.39g/cm3 |
|---|---|
| Boiling Point | 738ºC at 760mmHg |
| Molecular Formula | C28H28N2O5S |
| Molecular Weight | 504.59700 |
| Flash Point | 400.1ºC |
| Exact Mass | 504.17200 |
| PSA | 128.78000 |
| LogP | 6.37258 |
| Vapour Pressure | 7.22E-23mmHg at 25°C |
| Index of Refraction | 1.665 |
| Storage condition | 2-8℃ |