280578-49-6
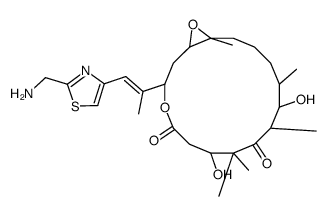
| Name | (1S,3S,7S,10R,11S,12S,16R)-3-[(E)-1-[2-(aminomethyl)-1,3-thiazol-4-yl]prop-1-en-2-yl]-7,11-dihydroxy-8,8,10,12,16-pentamethyl-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione |
|---|---|
| Synonyms |
4,17-Dioxabicyclo(14.1.0)heptadecane-5,9-dione,3-((1E)-2-(2-(aminomethyl)-4-thiazolyl)-1-methylethenyl)-7,11-dihydroxy-8,8,10,12,16-pentamethyl-,(1S,3S,7S,10R,11S,12S,16R)
(1S,3S(E),7S,10R,11S,12S,16R)-3-[2-(2-aminomethyl-thiazol-4-yl)-1-methyl-vinyl]-7,11-dihydroxy-8,8,10,12,16-pentamethyl-4,17-dioxa-bicyclo[14.1.0]heptadecane-5,9-dione 21-aminoepothilone B |
| Description | BMS 310705 (21-Aminoepothilone B) is an analog of Epothilone B (HY-17029), targeting to malignancies such as ovarian, renal, bladder, and lung carcinoma. BMS 310705 induces significant apoptosis via mitochondrial-mediated pathway[1]. |
|---|---|
| Related Catalog | |
| In Vitro | BMS 310705 (0.01-0.5 μM; 1 h) 诱导 OC-2 细胞凋亡[1]。 BMS 310705 (-0.5 μM; 1 h) 增加 caspase-9/-3 的活性,并导致细胞色素 c 释放[1]。 Apoptosis Analysis[1] Cell Line: OC-2 cells Concentration: 0.01 μM, 0.025 μM, 0.05 μM, and 0.5 μM Incubation Time: 1 hour; reincubation in drug-free media at 24-, 48-, 72-, and 96-h intervals Result: Induced maximal cell apoptosis at 0.05 μM, and exhibited time-dependent manner. |
| References |
[1]. Uyar D, et al. Apoptotic pathways of epothilone BMS 310705. Gynecol Oncol. 2003 Oct;91(1):173-8. |
| Density | 1.16 |
|---|---|
| Molecular Formula | C27H42N2O6S |
| Molecular Weight | 522.69700 |
| Exact Mass | 522.27600 |
| PSA | 163.51000 |
| LogP | 4.32810 |