114778-60-8
| Name | 2-amino-9-(3-hydroxypropoxy)-3H-purin-6-one |
|---|---|
| Synonyms |
9-(3-Hydroxypropoxy)guanine
9-(3-hydroxyprop-1-oxy)guanine 6H-Purin-6-one,2-amino-1,9-dihydro-9-(3-hydroxypropoxy) BRL44385 |
| Description | BRL44385 is a potent and selective inhibitor of the replication of herpes simplex virus types 1 and 2 (HSV-1 and HSV2), varicella zoster virus (VZV) and Epstein-Barr virus (EBV). |
|---|---|
| Related Catalog | |
| Target |
HSV-1 HSV-2 |
| In Vivo | BRL44385 is a selective antiherpesvirus agent. Following oral administration, BRL 55792 is very well absorbed and provides high and prolonged concentrations of BRL44385 in the blood. In mice 15 min and 60 min after administration of a single oral dose of BRL 55792, the concentrations of BRL44385 in the blood are considerably higher than those obtain after oral administration of either BRL44385 or BRL46720 and the bioavailability of BRL 44385 from oral BRL 55792 is 66% of that achieves with the same dose of the sodium salt of BRL44385 administered intravenously[1]. |
| Animal Admin | Mice[1] BRL44385 is administered as a single dose of 0.2 mmol/kg in 0.1 mL of 1% carboxymethylcellulose by oral gavage to female Balb/c mice weighing approximately 20 g. Food is withheld for 18 h prior to the start of the experiment. Blood is collected by cardiac puncture using heparinized syringes 15, 60, and 180 min after dosing. Equal volumes (0.2 mL) from three mice are pooled at each time point and 0.6 mL of 16% trichloroacetic acid is added. After centrifugation, 0.5 mL of supernatant is added to 0.1 mL of saturated aqueous NaHCO3 followed by the addition of 0.6 mL of 0.4 mM NH4OAc (pH 6.0) and the mixture analysed by HPLC. |
| References |
| Density | 1.77g/cm3 |
|---|---|
| Boiling Point | 543.6ºC at 760mmHg |
| Molecular Formula | C8H11N5O3 |
| Molecular Weight | 225.20500 |
| Flash Point | 282.5ºC |
| Exact Mass | 225.08600 |
| PSA | 120.04000 |
| Vapour Pressure | 1.2E-12mmHg at 25°C |
| Index of Refraction | 1.762 |
| Storage condition | 2-8℃ |
|
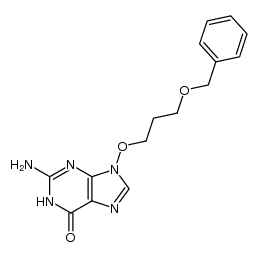
~32% 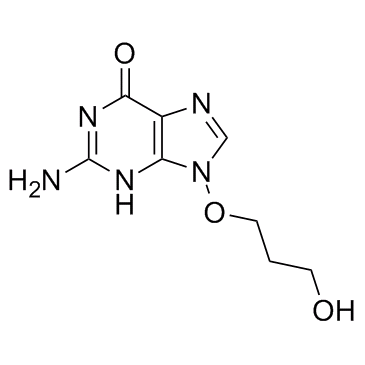
114778-60-8 |
| Literature: Beecham Group p.l.c. Patent: US4965270 A1, 1990 ; |
|
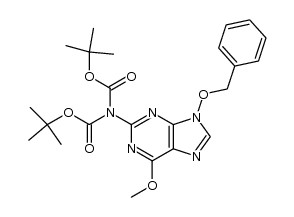
~91% 
114778-60-8 |
| Literature: Harnden, M. R.; Parkin, A.; Wyatt, P. G. Tetrahedron Letters, 1988 , vol. 29, # 6 p. 701 - 704 |
|
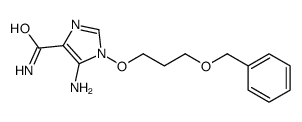
~61% 
114778-60-8 |
| Literature: Harnden, M. R.; Wyatt, P. G. Tetrahedron Letters, 1990 , vol. 31, # 15 p. 2185 - 2188 |
|
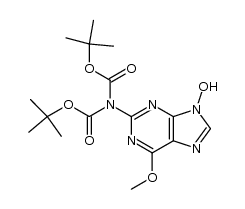
~% 
114778-60-8 |
| Literature: Harnden, M. R.; Wyatt, P. G. Tetrahedron Letters, 1990 , vol. 31, # 15 p. 2185 - 2188 |
|
~% 
114778-60-8 |
| Literature: Harnden, M. R.; Parkin, A.; Wyatt, P. G. Tetrahedron Letters, 1988 , vol. 29, # 6 p. 701 - 704 |
|
~% 
114778-60-8 |
| Literature: Harnden, M. R.; Parkin, A.; Wyatt, P. G. Tetrahedron Letters, 1988 , vol. 29, # 6 p. 701 - 704 |
|
~% 
114778-60-8 |
| Literature: Harnden, M. R.; Wyatt, P. G. Tetrahedron Letters, 1990 , vol. 31, # 15 p. 2185 - 2188 |