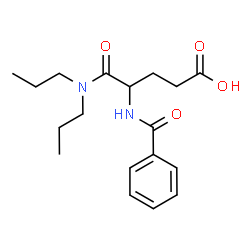
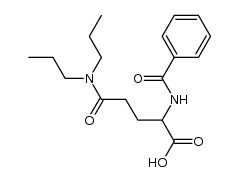
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
MA2805000
-
CHEMICAL NAME :
-
Glutaramic acid, 4-benzamido-N,N-dipropyl-, DL-
-
CAS REGISTRY NUMBER :
-
6620-60-6
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
12
-
MOLECULAR FORMULA :
-
C18-H26-N2-O4
-
MOLECULAR WEIGHT :
-
334.46
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
20 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 20,447,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1420 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 20,447,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5190 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Endocrine - other changes Skin and Appendages - corrosive (after topical exposure)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 5,203,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
8070 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
MIMEAO Minerva Medica. (Edizioni Minerva Medica, Casella Postale 491, I-10126 Turin, Italy) V.1- 1909- Volume(issue)/page/year: 58,3653,1967
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1480 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 20,447,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>5190 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Endocrine - other changes Skin and Appendages - corrosive (after topical exposure)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 5,203,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
MIMEAO Minerva Medica. (Edizioni Minerva Medica, Casella Postale 491, I-10126 Turin, Italy) V.1- 1909- Volume(issue)/page/year: 58,3653,1967 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
69 gm/kg/30D-C
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in bladder weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 5,203,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
546 gm/kg/26W-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Endocrine - changes in pituitary weight Blood - changes in leukocyte (WBC) count
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 5,203,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
327600 mg/kg/26W-C
-
TOXIC EFFECTS :
-
Endocrine - changes in adrenal weight Blood - changes in erythrocyte (RBC) count Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 5,203,1971 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1350 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 5,225,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5400 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 5,225,1971
|