145945-21-7
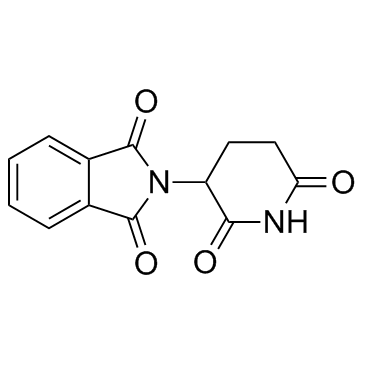
| Name | 2-[1-(hydroxymethyl)-2,6-dioxopiperidin-3-yl]isoindole-1,3-dione |
|---|---|
| Synonyms |
cps-11
unii-4a6c91xw54 |
| Description | CPS-11 (N-(Hydroxymethyl)thalidomide) a Thalidomide analogue, is a potent anti-cancer agent. CPS-11 inhibits NF-κB, activates NFAT, and repress cytokine expression through elevated ROS. CPS-11 exhibits a wider activity spectrum and higher potency against MM (multiple myeloma) cell lines[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | CPS-11 abrogates ability of bone marrow stromal cells (BMSCs) to induce proliferation of MM cells, confirming its ability to target tumor cells in the bone marrow microenvironment[1]. CPS-11 (0-200 μM, 0 or 4 h) shows virtually no effect on activation of p38 and only slight, transient activation of ATF2 and HSP27[2]. CPS-11 (0-100 μM, 24 or 48 h) was no toxic to H157 cells, WT MEFs or the p38α-/- MEFs at doses as high as 100 μM[2]. CPS-11 (50 μM, 24 or 48 h) does not induce apoptosis in H157 cells[2]. Western Blot Analysis[2] Cell Line: H157 cells, PC3 cells, HUVEC cells Concentration: 0, 1, 5, 10, 20, 50, 100 and 200 μM Incubation Time: 0, 15 min, 30 min, 1 h, 2 h, and 4 h Result: Showed virtually no effect on activation of p38 and only slight, transient activation of ATF2 and HSP27. Increased Akt phosphorylation within 15 minutes, and decreased Akt phosphorylation from 15 minutes to 4 hours. Cell Cytotoxicity Assay[2] Cell Line: H157 cells, wild-type (WT) mouse embryonic fibroblasts (MEF) and p38α-/- MEF Concentration: 0, 20, 50, and 100 μM Incubation Time: 24 or 48 hours Result: Showed no toxic to H157 cells at doses as high as 100 μM and an incubation time of 48 hours. had no toxic effect on either the WT MEFs or the p38α-/- MEFs at doses as high as 100 μM. Apoptosis Analysis[2] Cell Line: H157 cells Concentration: 50 μM Incubation Time: 24 or 48 hours Result: Did not induce apoptosis in H157 cells at either time point. |
| References |
| Molecular Formula | C14H12N2O5 |
|---|---|
| Molecular Weight | 288.25500 |
| Exact Mass | 288.07500 |
| PSA | 94.99000 |
|
~72% 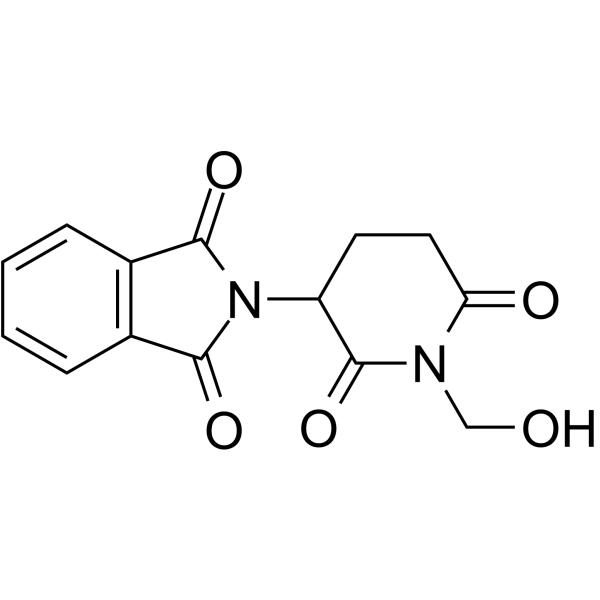
145945-21-7 |
| Literature: Stewart, Scott G.; Braun, Carlos J.; Polomska, Marta E.; Karimi, Mahdad; Abraham, Lawrence J.; Stubbs, Keith A. Organic and Biomolecular Chemistry, 2010 , vol. 8, # 18 p. 4059 - 4062 |
|
~67% 
145945-21-7 |
| Literature: Dos Santos, Jean Leandro; Lanaro, Carolina; Lima, Ldia Moreira; Gambero, Sheley; Franco-Penteado, Carla Fernanda; Alexandre-Moreira, Magna Suzana; Wade, Marlene; Yerigenahally, Shobha; Kutlar, Abdullah; Meiler, Steffen E.; Costa, Fernando Ferreira; Chung, Manchin Journal of Medicinal Chemistry, 2011 , vol. 54, # 16 p. 5811 - 5819 |