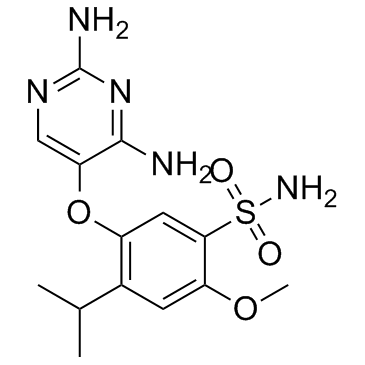
1015787-98-0
| Name | Gefapixant |
|---|---|
| Synonyms |
Gefapixant
AF-219 UNII:6K6L7E3F1L RO4926219 5-[(2,4-Diamino-5-pyrimidinyl)oxy]-4-isopropyl-2-methoxybenzenesulfonamide |
| Description | Gefapixant is an orally active P2X3 receptor (P2X3R) antagonist with IC 50s of ~30 nM versus recombinant hP2X3 homotrimers and 100-250 nM at hP2X2/3 heterotrimeric receptors. |
|---|---|
| Related Catalog | |
| Target |
IC50: ~30 nM (recombinant hP2X3 homotrimers), 100-250 nM (hP2X2/3 heterotrimeric receptors)[1]. |
| In Vitro | The aryloxy-pyrimidinediamine, Gefapixant (AF-219) is an orally active small molecule antagonist at human P2X3-containing receptors. The IC50 of Gefapixant has been reported as ~30 nM versus recombinant hP2X3 homotrimers and 100-250 nM at hP2X2/3 heterotrimeric receptors, potencies very similar to those reported for recombinant rat receptors, and it displays no inhibitory impact on any non-P2X3 subunit containing receptors (IC50 values>>10,000 nM at recombinant homotrimeric hP2X1, hP2X2, hP2X4, rP2X5 and hP2X7 channels)[1]. |
| In Vivo | In an adjuvant-induced rthritis model in rat (7d following intraplantar administration of complete Freund's adjuvant), AF-353 produces dose-dependent antihyperalgesia in weight-bearing asymmetry and von Frey filament mechanical tests; magnitude of effect is compared with that of the NSAID naproxen. In a rat model of knee osteoarthritis (14d following intra-articular administration of monoiodoacetate), Gefapixant (7d bid, orally; right) attenuates the weight bearing laterality with complete reversal of apparent hyperalgesia at the two higher doses[2]. |
| Animal Admin | Rats[2] A rodent model often employs for assessing potential for drug effect in osteoarthritis (OA) pain is based on intraarticular injection of monoiodoacetate (mIOA) into one knee joint of the rat. Progressive loss of chondrocytes leads to histological changes of the articular cartilage over subsequent weeks that resemble the changes which occur in human OA, leading to joint discomfort exemplified by a shift in the weight distribution (asymmetry) to favor the unaffected limb. To measure the effect of Gefapixant on the weight bearing laterality and apparent hyperalgesia, Gefapixant is given by intraplantar or oral administration to the rats, with different concentrations (6, 20, and 60 mg/kg) two times a day and continues up to a week[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 606.3±65.0 °C at 760 mmHg |
| Molecular Formula | C14H19N5O4S |
| Molecular Weight | 353.397 |
| Flash Point | 320.5±34.3 °C |
| Exact Mass | 353.115784 |
| LogP | 0.73 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.615 |
| Storage condition | 2-8℃ |