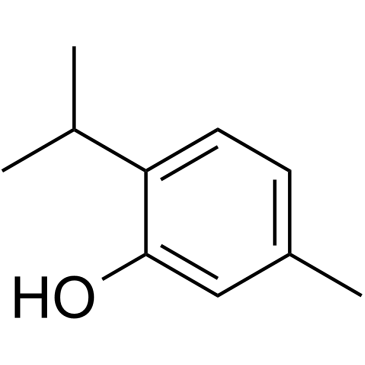
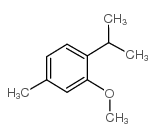
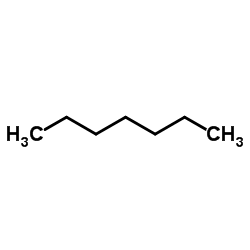
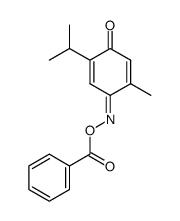
17302-61-3
| Name | (4E)-4-(Hydroxyimino)-2-isopropyl-5-methyl-2,5-cyclohexadien-1-on e |
|---|---|
| Synonyms |
2-methyl-5-(i-propyl)furan
5-methyl-2-isopropylfuran 2-isopropyl-5-methyl-p-benzoquinone 4-oxime Furan,2-methyl-5-(1-methylethyl) 2-methyl-5-isopropylfuran 2-Isopropyl-5-methyl-[1,4]benzochinon-4-oxim 5-methyl-2-(1-methylethyl)furan Thymochinon-oxim-(1) 2-isopropyl-5-methyl-[1,4]benzoquinone-4-oxime 2-isopropyl-5-methyl furan Poloxime |
| Description | Poloxime, a hydrolysis product of poloxin, is a non-ATP-competitive Plk1 inhibitor, with moderate Plk1 inhibitory activity. |
|---|---|
| Related Catalog | |
| Target |
PLK1 |
| In Vitro | Poloxime (100 μM) inhibits phosphopeptide binding to polo-box domain (PBD) of polo-like kinase 1 (Plk1)[2]. |
| References |
| Molecular Formula | C10H13NO2 |
|---|---|
| Molecular Weight | 179.21600 |
| Exact Mass | 179.09500 |
| PSA | 49.66000 |
| LogP | 1.92800 |
| Storage condition | 2-8℃ |
|
~% 
17302-61-3 |
| Literature: Dwyer; Mellor; Trikojus Journal and Proceedings of the Royal Society of New South Wales, 1932 , vol. 66, p. 315,319 |
|
~96% 
17302-61-3 |
| Literature: Rathore, R.; Kim, J. S.; Kochi, J. K. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1994 , # 19 p. 2675 - 2684 |
|
~% 
17302-61-3 |
| Literature: Klingstedt; Sundstroem Journal fuer Praktische Chemie (Leipzig), 1927 , vol. <2> 116, p. 311 |
|
~% 
17302-61-3 |
| Literature: Klingstedt; Sundstroem Journal fuer Praktische Chemie (Leipzig), 1927 , vol. <2> 116, p. 311 |
|
~% 
17302-61-3 |
| Literature: Hixon Journal of the American Chemical Society, 1923 , vol. 45, p. 2339 |
|
~% 
17302-61-3 |
| Literature: Sherk American Journal of Pharmacy and the Sciences Supporting Public Health (1937-1978), vol. 93, p. 214 Chem. Zentralbl., 1921 , vol. 92, # III p. 595 |
|
~% 
17302-61-3 |
| Literature: Goldschmidt; Schmid Chemische Berichte, 1884 , vol. 17, p. 2067 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |