6805-34-1
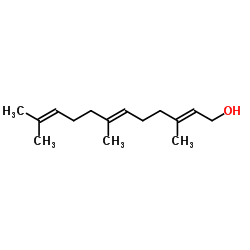
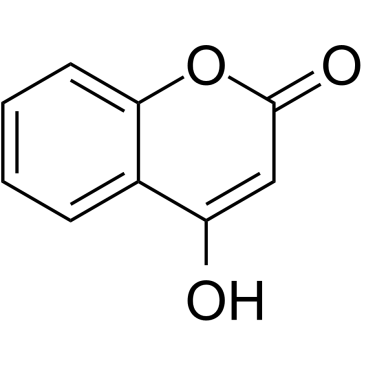
| Name | 4-hydroxy-3-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]chromen-2-one |
|---|---|
| Synonyms |
4-hydroxy-3-(3,7,11-trimethyl-2,6,10-dodecatrienyl)-2H-1-benzopyran-2-one
Ferulenol prenylated 4-hydroxycoumarin E,E-3-farnesyl-4-hydroxycoumarin |
| Description | Ferulenol, a sesquiterpene prenylated coumarin derivative, specifically inhibits succinate ubiquinone reductase at the level of the ubiquinonecycle. Ferulenol shows good antimycobacterial activity and haemorrhagic action[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Ferulenol inhibits the oxidative phosphorylation process by interacting with adenine nucleotide translocase (ANT) and the complex II of the respiratory chain. At low concentrations, ferulenol inhibited ATP synthesis by inhibition of the adenine nucleotide translocase without limitation of mitochondrial respiration. At higher concentrations, ferulenol inhibited oxygen consumption. Ferulenol caused specific inhibition of succinate ubiquinone reductase without altering succinate dehydrogenase activity of the complex II[1]. Ferulenol inhibits succinate ubiquinone reductase(SQR) activity in a concentration-dependent manner and is as effective as thenoyltrifluoroacetone (TTFA)[1]. |
| References |
| Density | 1.07g/cm3 |
|---|---|
| Boiling Point | 492.8ºC at 760 mmHg |
| Molecular Formula | C24H30O3 |
| Molecular Weight | 366.49300 |
| Flash Point | 161.7ºC |
| Exact Mass | 366.21900 |
| PSA | 50.44000 |
| LogP | 6.46030 |
| Index of Refraction | 1.559 |
| HS Code | 2932209090 |
|---|
|
~% 
6805-34-1 |
| Literature: Gebauer, Markus Bioorganic and Medicinal Chemistry, 2007 , vol. 15, # 6 p. 2414 - 2420 |
|
~9% 
6805-34-1 |
| Literature: Gebauer, Markus Bioorganic and Medicinal Chemistry, 2007 , vol. 15, # 6 p. 2414 - 2420 |
|
~% 
6805-34-1 |
| Literature: Appendino; Cravotto; Nano; Palmisano Synthetic Communications, 1992 , vol. 22, # 15 p. 2205 - 2212 |
|
~4% 
6805-34-1 |
| Literature: Cravotto, Giancarlo; Nano, Gian Mario; Palmisano, Giovanni; Tagliapietra, Silvia Synthesis, 2003 , # 8 p. 1286 - 1291 |
| Precursor 5 | |
|---|---|
| DownStream 1 | |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |