CCG 203769 Suppliers
Total count: 3Updated Date: 2024-12-06 14:14:50

- China(Mainland)
- Contact: Yang
- Phone: 0086-021-52280163
- Email: sales@echemcloud.com
- Website: http://www.echemcloud.com
- Product Name: CCG 203769?
- Updated Date: 2024-07-15 12:35:11
- Purity: 99.0%
- More information
Inquiry

- China(Mainland)
- Contact: Tony Lai
- Phone: 13564518121
- Email: order@dcchemicals.com
- Website: http://www.biomedgen.cn
- Product Name: CCG 203769
- Updated Date: 2025-08-20 09:10:32
- Purity: 98.9%
- More information
¥Inquiry
1g

- China(Mainland)
- Contact: Tony Cao
- Phone: 13564518121
- Email: sales@dcchemicals.com
- Website: http://www.dcchemicals.com
- Product Name: CCG 203769
- Updated Date: 2025-08-27 07:27:33
- Purity: 98.0%
- More information
¥850.0
100mg
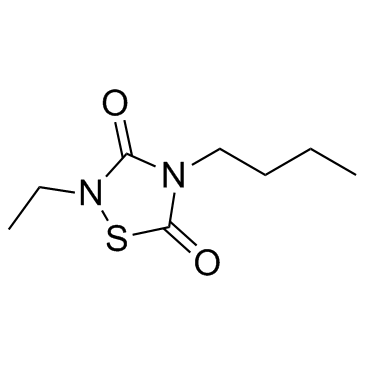
CCG 203769
- CAS Number: 410074-60-1
- Molecular Formula: C8H14N2O2S
- Molecular Weight: 202.274
Price: $350/5mg
Reference only. More CCG 203769 price
Related product suppliers
Check more product suppliers