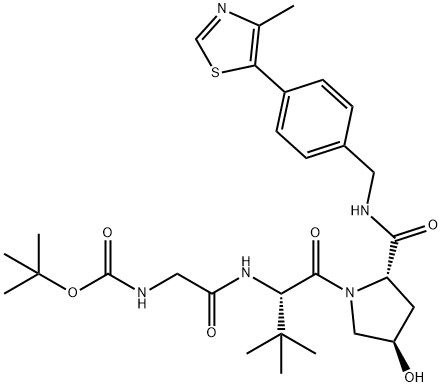
VH032-NH-CO-CH2-NHBoc Suppliers
Total count: 1
- China(Mainland)
- Contact: Yang
- Phone: 0086-021-52280163
- Email: sales@echemcloud.com
- Website: http://www.echemcloud.com
- Product Name: VH032-NH-CO-CH2-NHBoc
- Updated Date: 2024-07-15 12:33:25
- Purity: 98.0%
- More information
Inquiry
VH032-NH-CO-CH2-NHBoc
- CAS Number: 2010986-19-1
- Molecular Formula: C29H41N5O6S
- Molecular Weight: 587.73
Related product suppliers
Check more product suppliers