CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DG9895000
-
CHEMICAL NAME :
-
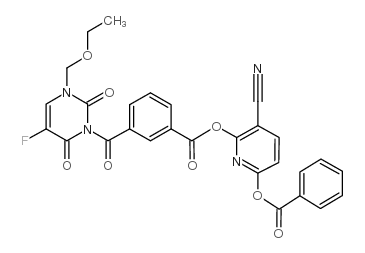
Benzoic acid, 3-((3-(ethoxymethyl)-5-fluoro-3,6-dihydro-2,6-dioxo-1 (2H)-pyrimidinyl) carbonyl)-, 6-(benzoyloxy)-3-cyano-2-pyridinyl ester
-
CAS REGISTRY NUMBER :
-
110690-43-2
-
LAST UPDATED :
-
199503
-
DATA ITEMS CITED :
-
13
-
MOLECULAR FORMULA :
-
C28-H19-F-N4-O8
-
MOLECULAR WEIGHT :
-
558.51
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1850 mg/kg
-
TOXIC EFFECTS :
-
Blood - changes in spleen Skin and Appendages - hair Nutritional and Gross Metabolic - body temperature decrease
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Gastrointestinal - hypermotility, diarrhea
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Blood - changes in spleen Nutritional and Gross Metabolic - body temperature decrease
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Olfaction) - effect, not otherwise specified Gastrointestinal - hypermotility, diarrhea Blood - changes in spleen
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3640 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Blood - changes in erythrocyte (RBC) count Blood - changes in leukocyte (WBC) count Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3640 mg/kg/1Y-I
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Blood - changes in spleen Blood - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
7280 mg/kg/1Y-I
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - changes in chlorine Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - dehydrogenases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
2730 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
520 mg/kg
-
SEX/DURATION :
-
female 17-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
65 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
Micronucleus test
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Rodent - hamster Ovary
-
DOSE/DURATION :
-
60 mg/L
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 18(Suppl 3),11,1993
|