HYDROXYPROPYL CELLULOSE, 6-10 MPAS
Published date: 2017-09-12 19:56:04
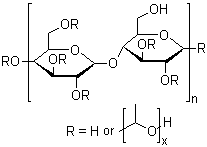
| Name | HYDROXYPROPYL CELLULOSE, 6-10 MPAS | CAS# | 78214-41-2 | |
|---|---|---|---|---|
| Price | ¥Inquiry/1kg | Purity | 99.0% | |
| Stocking Period |
Inquiry | Stock | Inquiry |
| Detail
Product Information
Chemical & Physical Properties: CAS Number: 78214-41-2 Name: HYDROXYPROPYL CELLULOSE, 6-10 MPAS Molecular Formula: Molecular Weight: Density: N/A Boiling Point: N/A Melting Point: N/A Flash Point: N/A |