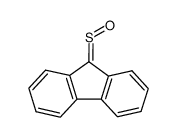
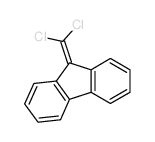
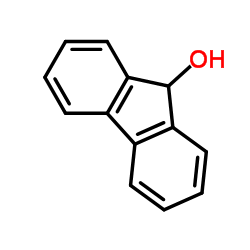
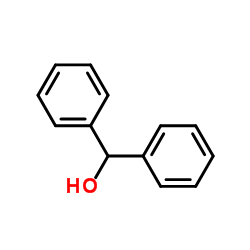
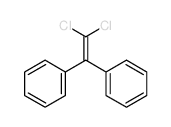
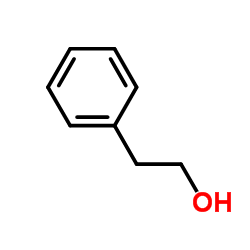
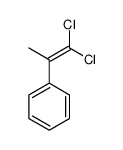
|
~65% |
|
~14% |
|
~% |
|
~% |
|
~% |
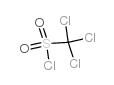
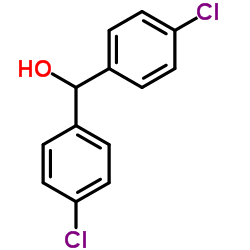
![Benzene,1,1'-[[(trichloromethyl)sulfonyl]methylene]bis[4-chloro Structure](https://image.chemsrc.com/caspic/194/66229-26-3.png)