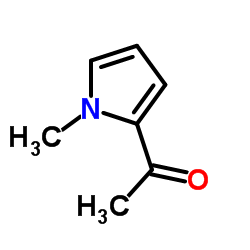
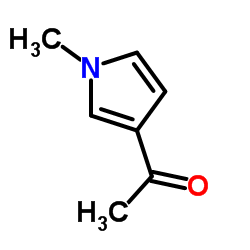
|
~% |
|
~% |
|
~% |
|
~78% |
|
~% |
|
~13% |
|
~44% |
|
~41% |
|
~% |
|
~% |
|
~% |
|
~43% |
|
~% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~64% |
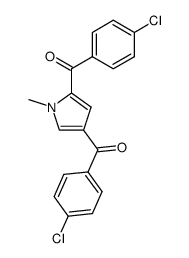
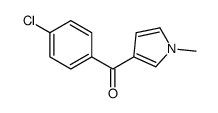
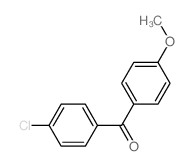
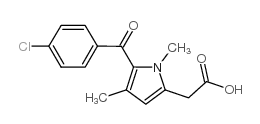
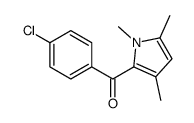
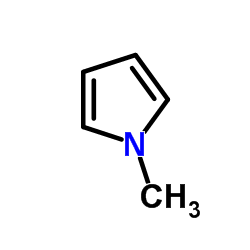
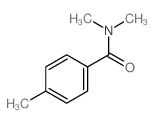
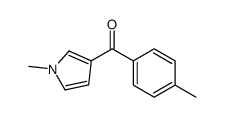
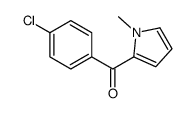
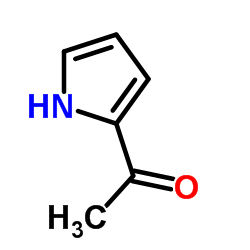


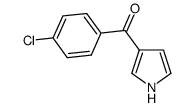
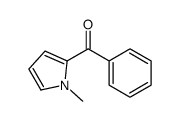

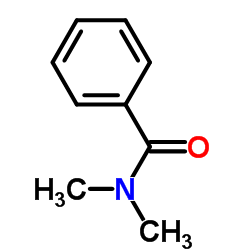
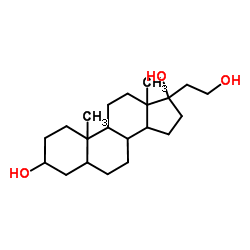
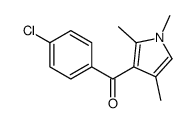
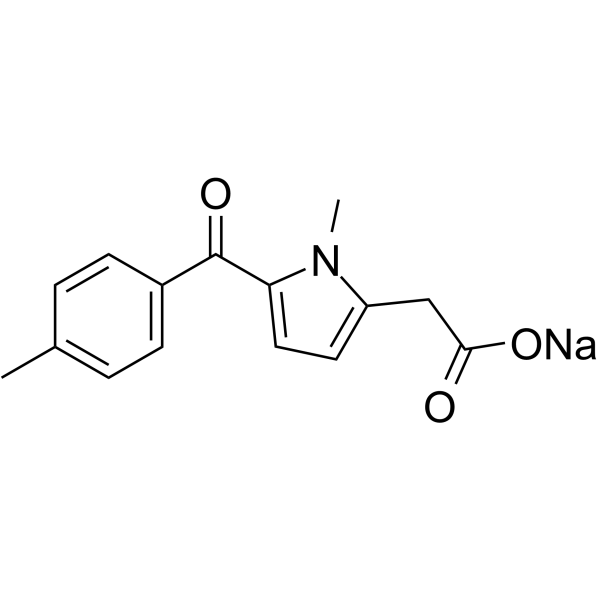

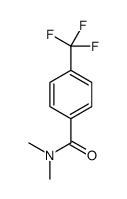
![(1-methylpyrrol-3-yl)-[4-(trifluoromethyl)phenyl]methanone Structure](https://image.chemsrc.com/caspic/494/62356-33-6.png)