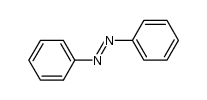
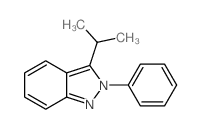
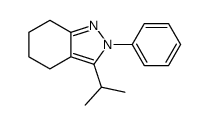
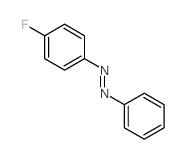
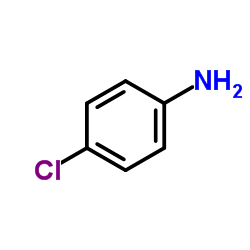
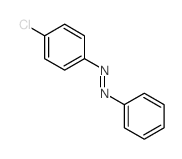
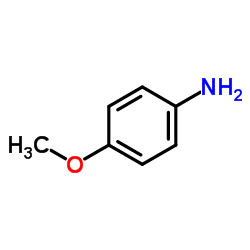
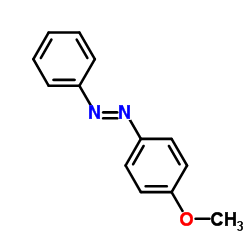
|
~44% |
|
~58% |
|
~% |
|
~% |
|
~46% |
|
~40% |
|
~69% |
|
~13% |