A biosynthetic pathway generating 12-hydroxy-5,8,14-eicosatrienoic acid from arachidonic acid is active in mouse skin microsomes.
Liping Du, Valery Yermalitsky, David L Hachey, Setti G Jagadeesh, John R Falck, Diane S Keeney
Index: J. Pharmacol. Exp. Ther. 316(1) , 371-9, (2006)
Full Text: HTML
Abstract
The epidermis expresses cyclooxygenases, lipoxygenases, and cytochromes P450, which utilize arachidonic acid to generate a diverse array of lipid mediators affecting epidermal cellular differentiation and functions. Recent studies show that mouse epidermis expresses CYP2B19, a keratinocyte-specific epoxygenase that generates 11,12- and 14,15-epoxyeicosatrienoic (EET) acids from arachidonate. We studied CYP2B19-dependent metabolism in mouse epidermal microsomes, reconstituted in the presence of [1-(14)C]arachidonic acid. The majority of the (14)C products formed independently of NADPH, indicative of robust epidermal cyclooxygenase and lipoxygenase activities. We studied two NADPH-dependent products generated in a highly reproducible manner from arachidonate. One of these (product I) coeluted with the CYP2B19 product 14,15-EET on a reversed-phase high-performance liquid chromatography (HPLC) system; there was no evidence for other regioisomeric EET products. Further analyses proved that product I was not an epoxy fatty acid, based on different retention times on a normal-phase HPLC system and failure of product I to undergo hydrolysis in acidic solution. We analyzed purified epidermal (14)C products by liquid chromatography negative electrospray ionization mass spectrometry. Structures of the NADPH-dependent products were confirmed to be 12-oxo-5,8,14-eicosatrienoic acid (I) and 12-hydroxy-5,8,14-eicosatrienoic acid (II). This was the first evidence for a 12-hydroxy-5,8,14-eicosatrienoic acid biosynthetic pathway in mouse epidermis. Epidermal microsomes also generated 12-hydroperoxy, 12-hydroxy, and 12-oxo eicosatetraenoic acids from arachidonate, possible intermediates in the 12-hydroxy-5,8,14-eicosatrienoic acid biosynthetic pathway. These results predict that hydroxyeicosatrienoic acids are synthesized from arachidonate in human epidermis. This would have important implications for human skin diseases given the known pro- and anti-inflammatory activities of stereo- and regioisomeric hydroxyeicosatrienoic acids.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
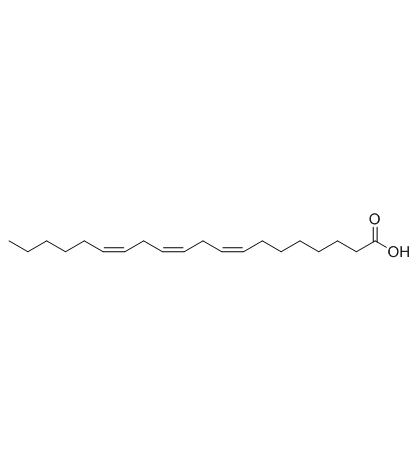 |
cis-8,11,14-Eicosatrienoic Acid
CAS:1783-84-2 |
C20H34O2 |
|
HMDB: a knowledgebase for the human metabolome.
2009-01-01 [Nucleic Acids Res. 37(Database issue) , D603-10, (2009)] |
|
The human serum metabolome.
2011-01-01 [PLoS ONE 6(2) , e16957, (2011)] |
|
Development and application of a comparative fatty acid anal...
2015-01-01 [Clin. Chim. Acta 438 , 126-34, (2014)] |
|
Lipidomics reveals a remarkable diversity of lipids in human...
2010-11-01 [J. Lipid Res. 51(11) , 3299-305, (2010)] |
|
Is the origin of atopy linked to deficient conversion of ome...
1989-09-01 [J. Am. Acad. Dermatol. 21(3 Pt 1) , 557-63, (1989)] |