Inhibition of matrix metalloproteinases by peptidyl hydroxamic acids.
S Odake, Y Morita, T Morikawa, N Yoshida, H Hori, Y Nagai
Index: Biochem. Biophys. Res. Commun. 199 , 1442-6, (1994)
Full Text: HTML
Abstract
Synthetic inhibitors of interstitial collagenase, tri- and tetrapeptidyl hydroxamic acids, have been developed and tested for their inhibitory activities against human matrix metalloproteinases. A water soluble inhibitor, p-NH2-Bz-Gly-Pro-D-Leu-D-Ala-NHOH (FN-439) inhibited interstitial and granulocyte collagenases, granulocyte gelatinase and skin fibroblast stromelysin with IC50 of 1 x 10(-6) M, 3.0 x 10(-5) M and 1.5 x 10(-4), respectively, but not thermolysin and serine proteinases. FN-439 was found to retain its inhibitory activity against matrix metalloproteinases even after prolonged incubation with pronase or human granulocyte elastase, indicating a favorite candidate of the inhibitor to modulate metalloproteinase activities in vivo.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
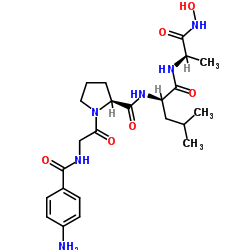 |
4-Abz-Gly-Pro-D-Leu-D-Ala-NHOH trifluoroacetate salt
CAS:124168-73-6 |
C23H34N6O6 |
|
Impaired Focal Adhesion Kinase-Grb2 Interaction during Eleva...
2015-01-01 [Int. J. Mol. Sci. 16 , 15659-69, (2015)] |
|
Vertebrate collagenase inhibitor. II. Tetrapeptidyl hydroxam...
1991-06-01 [Chem. Pharm. Bull. Tokyo 39 , 1489-1494, (1991)] |
|
Modeling of inhibitor-metalloenzyme interactions and selecti...
1998-04-01 [Proteins 31 , 42-60, (1998)] |
|
Effects of a combination of an antibacterial agent (ofloxaci...
1996-12-01 [J. Endod. 22 , 668-673, (1996)] |
|
Inhibition of corneal ulceration by tetrapeptidyl hydroxamic...
1995-01-01 [Jpn. J. Ophthalmol. 39 , 35-42, (1995)] |