Identification of (3S, 9R)- and (3S, 9S)-megastigma-6,7-dien-3,5,9-triol 9-O-beta-D-glucopyranosides as damascenone progenitors in the flowers of Rosa damascena Mill.
Masayuki Suzuki, Shigetaka Matsumoto, Masaya Mizoguchi, Satoshi Hirata, Kazuteru Takagi, Ikue Hashimoto, Yumiko Yamano, Masayoshi Ito, Peter Fleischmann, Peter Winterhalter, Tetuichiro Morita, Naoharu Watanabe
Index: Biosci. Biotechnol. Biochem. 66(12) , 2692-7, (2002)
Full Text: HTML
Abstract
The progenitors of damascenone (1), the most intensive C13-norisoprenoid volatile aroma constituent of rose essential oil, were surveyed in the flowers of Rosa damascena Mill. Besides 9-O-beta-D-glucopyranosyl-3-hydroxy-7,8-didehydro-beta-ionol (4b), a stable progenitor already isolated from the residual water after steam distillation of flowers of R. damascena Mill., two labile progenitors were identified to be (3S, 9R)- and (3S, 9S)-megastigma-6,7-dien-3,5,9-triol 9-O-beta-D-glucopyranosides (2b) based on their synthesis and HPLC-MS analytical data. Compound 2b gave damascenone (1), 3-hydroxy-beta-damascone (3) and 4b upon heating under acidic conditions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
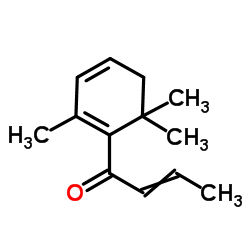 |
Damascenone
CAS:23696-85-7 |
C13H18O |
|
Instrumental and sensory approaches for the characterization...
2008-06-01 [Chem. Biodivers. 5(6) , 1170-83, (2008)] |
|
Identification and quantification of aroma-active components...
2002-03-27 [J. Agric. Food Chem. 50(7) , 2016-21, (2002)] |
|
Simultaneous determination of E-2-nonenal and beta-damasceno...
2004-04-02 [J. Chromatogr. A. 1032(1-2) , 17-22, (2004)] |
|
Use of solid-liquid distribution coefficients to determine r...
2001-10-05 [J. Chromatogr. A. 931(1-2) , 31-9, (2001)] |
|
Volatile compounds responsible for aroma of Jutrzenka liquer...
2011-10-21 [J. Chromatogr. A. 1218(42) , 7566-73, (2011)] |