tRNA aminoacylation by arginyl-tRNA synthetase: induced conformations during substrates binding.
B Delagoutte, D Moras, J Cavarelli
Index: EMBO J. 19 , 5599-5610, (2000)
Full Text: HTML
Abstract
The 2.2 A crystal structure of a ternary complex formed by yeast arginyl-tRNA synthetase and its cognate tRNA(Arg) in the presence of the L-arginine substrate highlights new atomic features used for specific substrate recognition. This first example of an active complex formed by a class Ia aminoacyl-tRNA synthetase and its natural cognate tRNA illustrates additional strategies used for specific tRNA selection. The enzyme specifically recognizes the D-loop and the anticodon of the tRNA, and the mutually induced fit produces a conformation of the anticodon loop never seen before. Moreover, the anticodon binding triggers conformational changes in the catalytic center of the protein. The comparison with the 2.9 A structure of a binary complex formed by yeast arginyl-tRNA synthetase and tRNA(Arg) reveals that L-arginine binding controls the correct positioning of the CCA end of the tRNA(Arg). Important structural changes induced by substrate binding are observed in the enzyme. Several key residues of the active site play multiple roles in the catalytic pathway and thus highlight the structural dynamics of the aminoacylation reaction.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
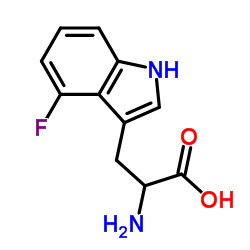 |
4-Fluorotryptophan
CAS:25631-05-4 |
C11H11FN2O2 |
|
Fluorine-19 nuclear magnetic resonance spectroscopic study o...
1994-05-03 [Biochemistry 33(17) , 5238-45, (1994)] |
|
Atomic mutations at the single tryptophan residue of human r...
1999-08-17 [Biochemistry 38(33) , 10649-59, (1999)] |
|
Incorporation of tryptophan analogues into staphylococcal nu...
1998-06-23 [Biochemistry 37(25) , 8938-46, (1998)] |
|
Phosphorescence and optically detected magnetic resonance ch...
1998-06-23 [Biochemistry 37(25) , 8954-64, (1998)] |
|
Intracellular uptake and inhibitory activity of aromatic flu...
2008-09-01 [ChemMedChem 3 , 1449, (2008)] |