Enantiomeric separation of basic drugs using N-benzyloxycarbonylglyclyl-L-proline as counter ion in methanol.
N H Huynh, A Karlsson, C Pettersson
Index: J. Chromatogr. A. 705(2) , 275-87, (1995)
Full Text: HTML
Abstract
Direct separation of enantiomeric amines using mainly N-benzyloxycarbonylglycyl-L-proline (L-ZGP) but also N-benzyloxycarbonylglyclglcyl-L-proline (L-ZGGP) as the chiral counter ion in methanol is described. The solid phase was Hypercarb porous graphitic carbon. Several amines of pharmacological interest (e.g., alprenolol, sotalol, terbutaline, promethazine and trimipramine) were separated with high enantioselectivity (alpha = 1.16-1.98) using L-ZGP and L-ZGGP as chiral selectors. In accordance with ion-pair chromatography, the retention of the enantiomeric amines was found to increase with increasing concentration of the anionic form of L-ZGP. Addition of a base (sodium hydroxide or an alkylamine) in excess of L-ZGP gave rise to a decrease in retention and enantioselectivity. The enantioselective retention was also affected by adding 2-propanol or acetonitrile to the mobile phase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
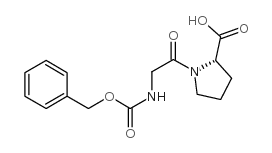 |
Z-Gly-Pro-OH
CAS:1160-54-9 |
C15H18N2O5 |
|
Determination of (R)- and (S)-propranolol in plasma by high-...
[J. Chromatogr. A. 494 , 157-71, (1989)] |
|
Enantioselective high-performance liquid chromatographic det...
[J. Chromatogr. A. 620(2) , 217-24, (1993)] |
|
Chiral separation of amines with N-benzoxycarbonylglycyl-L-p...
2003-01-17 [J. Chromatogr. A. 984(2) , 261-71, (2003)] |
|
Possible involvement of aminopeptidase, an ecto-enzyme, in t...
1985-10-30 [Biochim. Biophys. Acta 847(1) , 67-76, (1985)] |
|
Structures of prolyl oligopeptidase substrate/inhibitor comp...
2001-01-12 [J. Biol. Chem. 276(2) , 1262-6, (2001)] |